
Mg To Meq Calculator
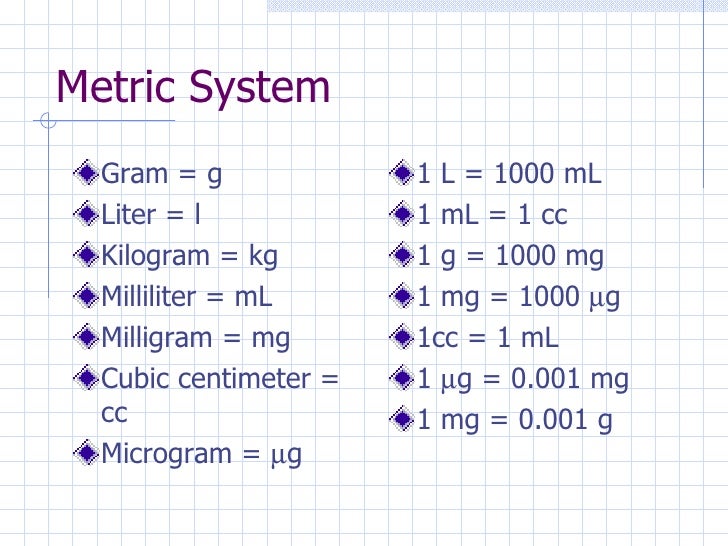
A milligram, abbreviated mg, is a metric unit of mass or weight defined as one thousandth of a gram. A milliequivalent is a measure of the quantity of ions in an electrolyte fluid. One milliequivalent is one thousandth of one mole of charges and is represented by the symbol mEq.
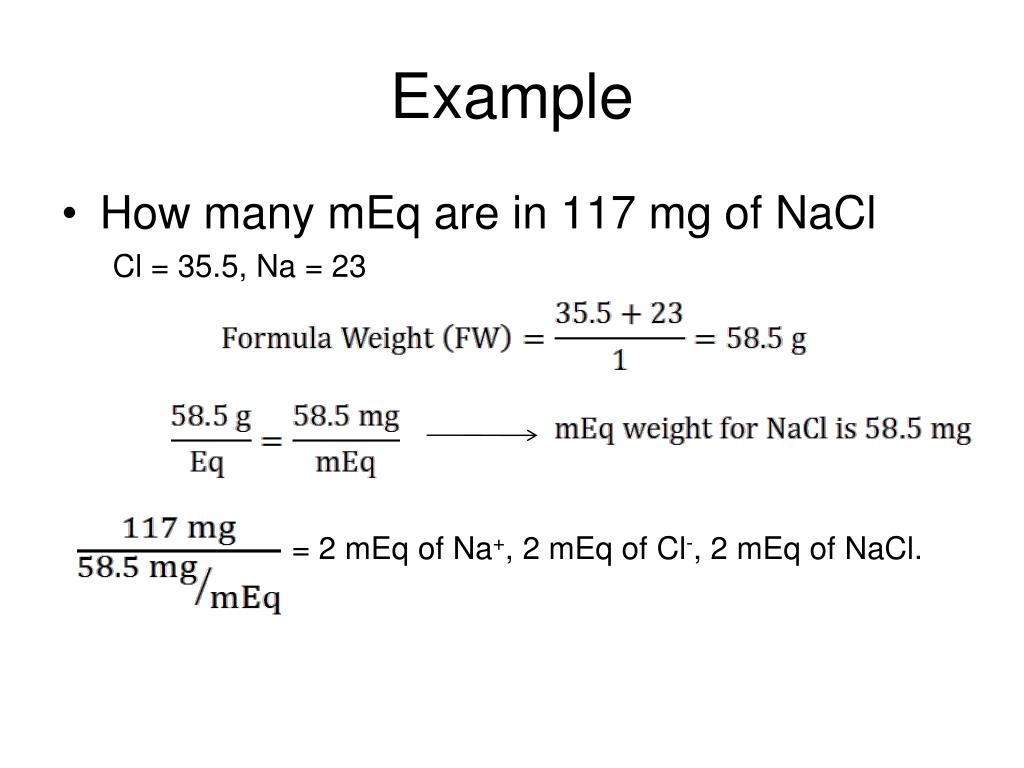
PPT Millis (Equivalents and Moles) PowerPoint Presentation, free download ID1418656
mg = 2 * (22.99 / 1) = 2 * 22.99 = 45.98 mg. Therefore, 2 mEq of sodium ions is equivalent to 45.98 mg of sodium. More Calculator. 10 mEq to mg: 20 mEq to mg: potassium mg to mEq: potassium mEq to mg: Reference:-periodic table in wikipedia: visit: pubchem.ncbi.nlm.nih.gov in periodic table: visit: About; Terms;

meq与mg换算,meq和mg怎么转化,毫克当量与毫克的换算_大山谷图库
The order of magnitude of dose is 1.0 meq Mg/kg on day 1, and 0.3 to 0.5 mEq/kg per day for 3 to 5 days. In emergencies such as convulsions or ventricular arrhythmias, a bolus injection of 1.0 gm (8.1 meq) of MgSO4 is indicated. Therapy of Mg deficiency in the presence of renal insufficiency requires smaller doses and frequent monitoring.

RUMUS KOREKSI HYPOKALEMIA DAN... Serbaserbi Perawat
The Nutrition and Food Web Archive is Your #1 Source for Free Nutrition and Food-Related Resources on the Internet. Chris Theberge developed NAFWA for clinical dietitians, dietetic interns, and anyone interested in the field of nutrition.. Formula: mEq= mg/atomic weight * valence; Minerals: Atomic Weight: Valence: Calcium : 40: 2: C hlorine.
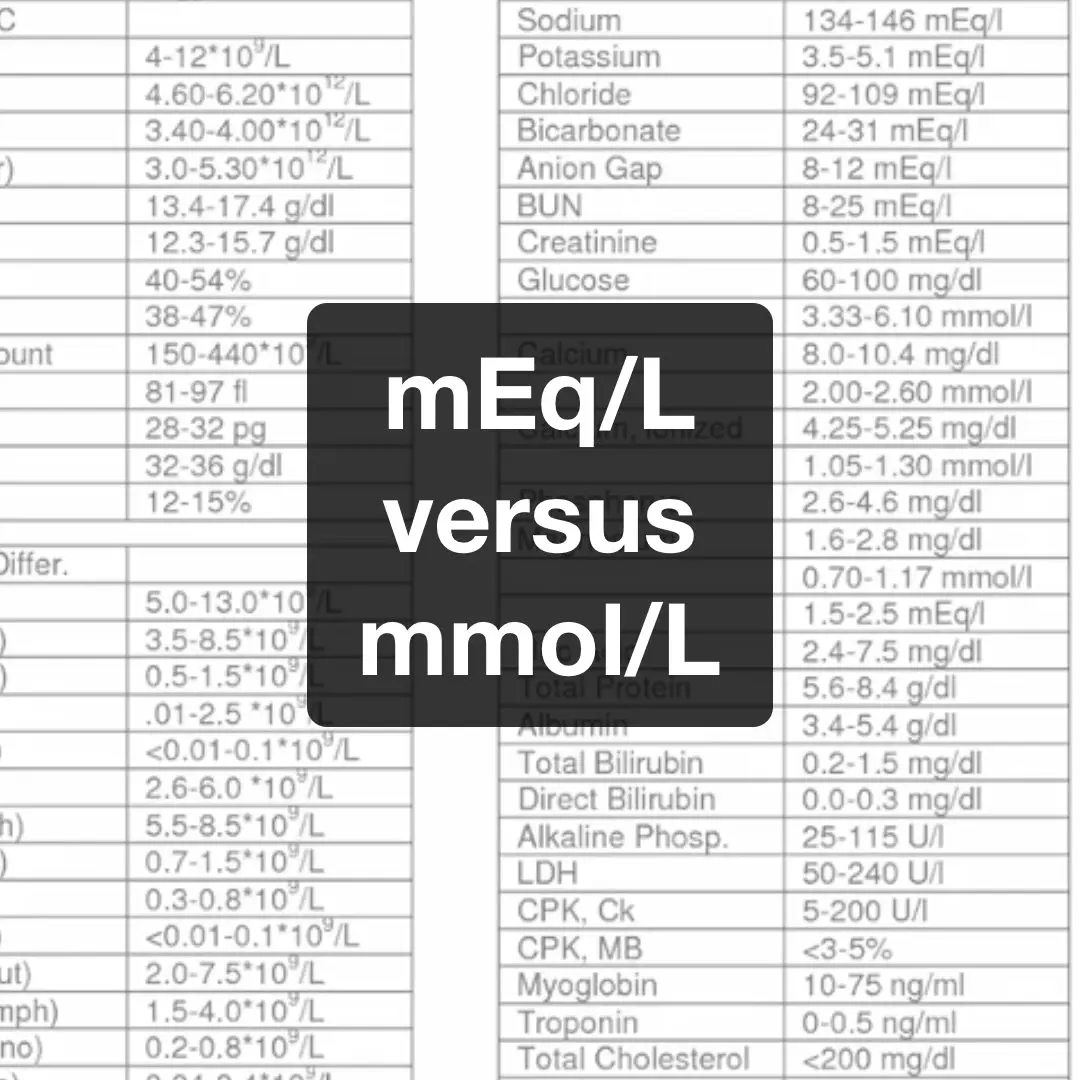
Milliequivalents (mEq) versus Millimole (mmol) RK.MD
Perhitungan. Untuk mengkonversi satu meq potassium ke mg, kita perlu mengetahui berapa berat atom potassium dan menghitung berapa mg yang setara dengan satu meq. Berat atom potassium adalah sekitar 39.1 g/mol. Karena satu meq adalah setara dengan 1/1000 mol, maka berat satu meq potassium adalah sekitar 0,0391 g atau 39,1 mg.

Ctm Berapa Mg Login pages Info
1 10-ml ampule 50% MgSO4 = 5 grams Mg = 40.6 mEq Mg. 1 gram Mg = 8.12 mEq Mg. 1 mEq Mg = 0.5 mmol Mg = 12.3 mg Mg. 1 mEq/dL Mg = 1.2 mg/dL. This page was first uploaded to The Magnesium Web Site on February 13, 2002.
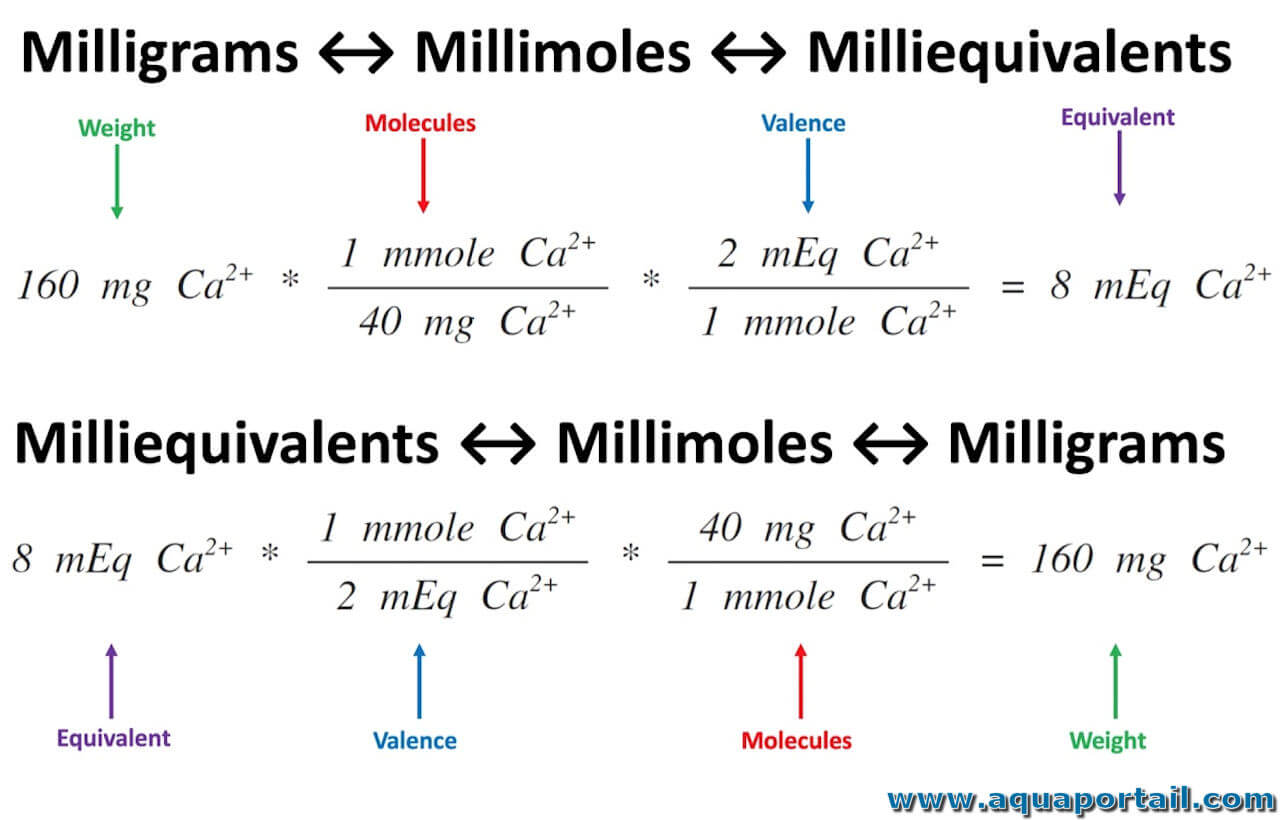
Milliéquivalent définition et explications
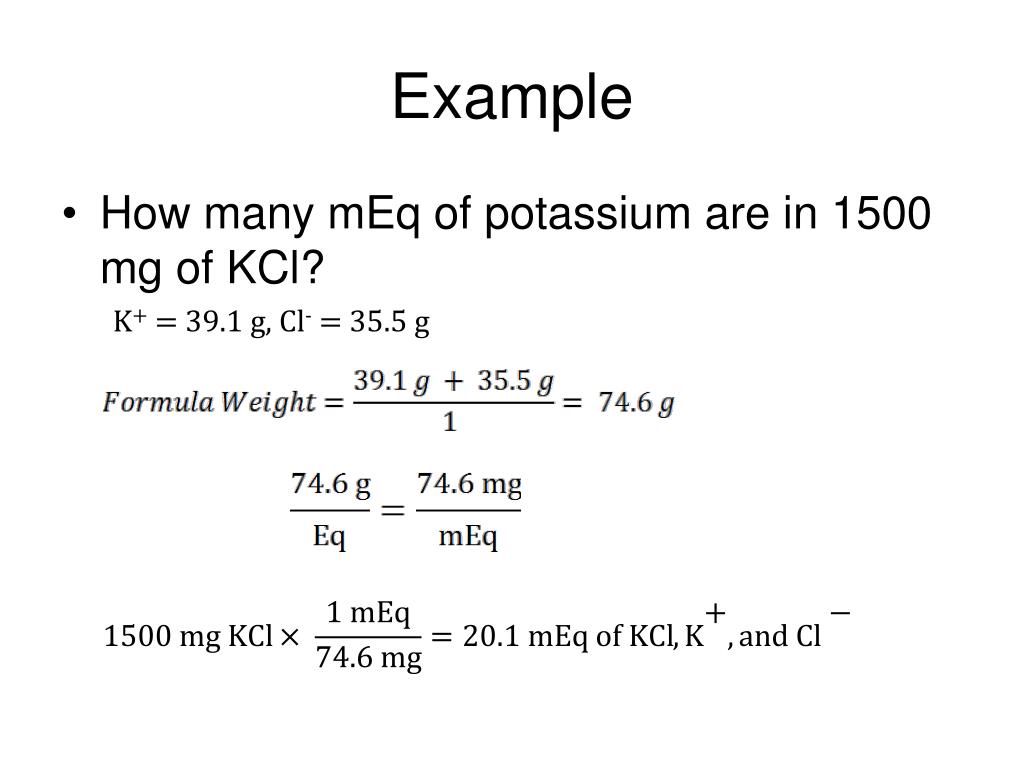
Answer. Step 1: Determine the valence. Potassium has a valence of 1. Step 3: Plug the valence, molecular weight and valence values in the mg to mEq equation below. mg = (mEq x molecular weight)/valence. mg = (20 x 39)/1. mg = 780.

Meylon 84 BP Injection 25 ml Farmaku
The conversion factor between the units mol/ z, eq, val, and g-equivalent is one, meaning 1 mmol/L ⋅ 1/ z = 1 meq/L = 1 mval/L = 1 мг-экв/л = 1 mg-eq/L. For converting an equivalent concentration ceq into the corresponding ↑ molar concentration c, divide the equivalent concentration by the valency z, i.e. ceq / z = c.
KSR 600 Mg 10 Tablet Alodokter Shop
1 g NaCl = 393 mg Na = 17 mEq Na. 1000 mL saline = 9 g NaCl = 3.5 g Na = 151 mEq Na. 1000 mL lactated Ringer's solution = 3 g Na = 130 mEq Na. 1 g KCL = 524 mg K = 13 mEq K. 1 g calcium gluconate = 93 mg Ca = 4.6 mEq Ca. 1 g CaCO3 = 400 mg Ca = 20 mEq Ca. 1 mL Fe dextran (Imferon) = 50 mg Fe. 1 g MgSO4 = 98 mg elemental Mg.
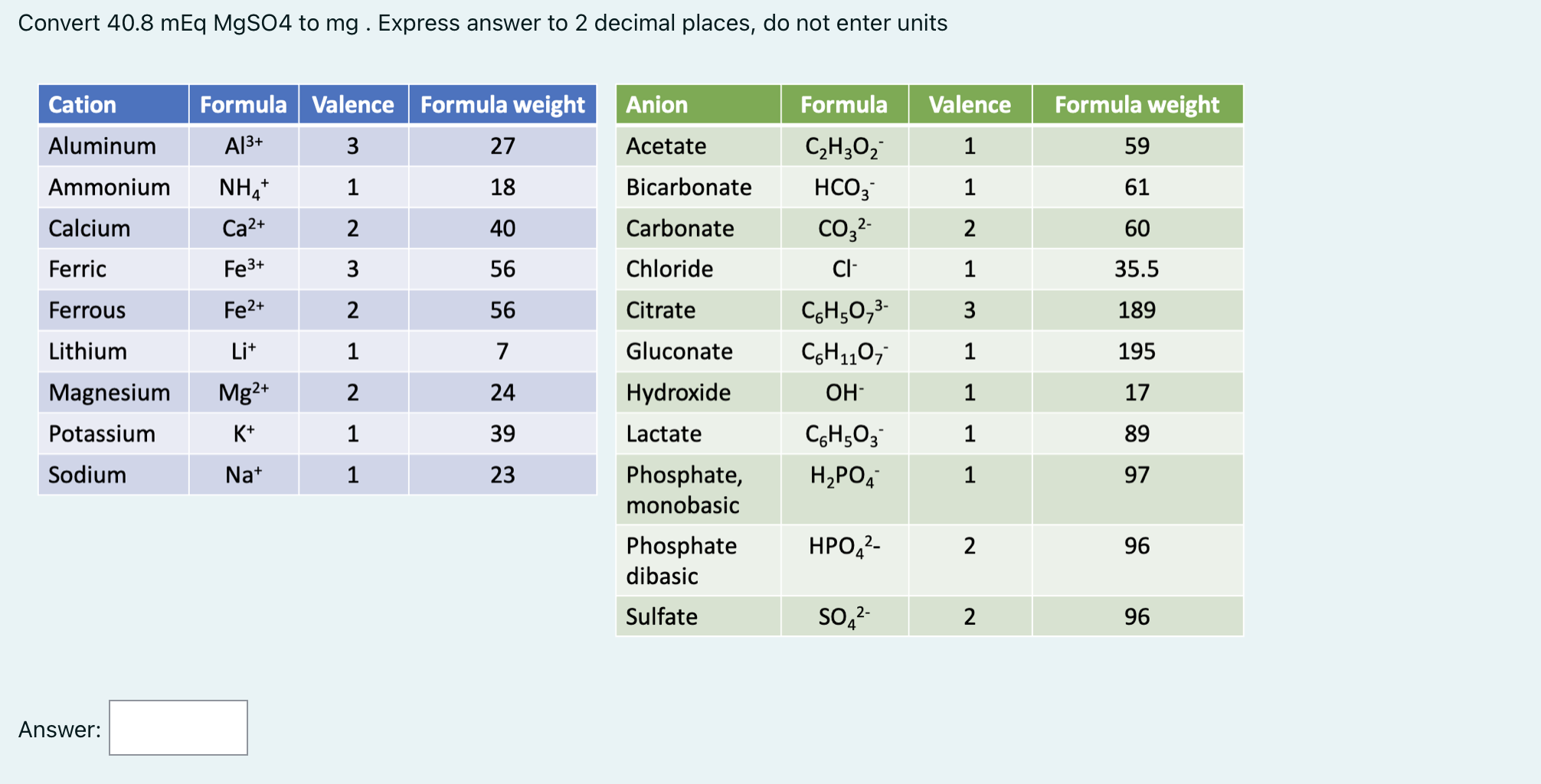
Solved Convert 40.8 mEq MgSO4 to mg. Express answer to 2
The formula used in this calculator is: meq = mg/ (atomic weight of potassium). Here, mg stands for milligrams of potassium and meq for milliequivalents. The atomic weight of potassium is a constant, approximately 39.1. By substituting the given mg into this formula, the calculator swiftly determines the equivalent meq.
PPT Millis (Equivalents and Moles) PowerPoint Presentation, free download ID1418656
From the above equation, and with the valence of elemental potassium being 1, you have mEq = [(58.65 mg)(1)]/39.1 mg/mmol = 1.5 mEq. A solution contains 30 mg of NaCl (table salt) per 400 mL of solution. Express the solution in terms of milliequivalents per liter (mEq/L). (Note: the molecular weight of NaCl is 58.44 g/mol.)
Como você calcula mEq de MG?
8.4% concentration = 50 mEq in 50 mL. 8.4% concentration supplies 84 mg/mL, which also consists 1 mEq/mL for each sodium and bicarbonate. One ampoule of 50 ml contains 50 mEq sodium and 50 mEq bicarbonate to a total of 100 mEq/50 mL and corresponds to 2000 mosm per liter. This formulation is a hypertonic solution and can raise serum sodium.

How to Convert Meq to Mg for Potassium MarckruwOconnor
Next, determine the molecular weight. The molecular weight is found to be 40 mg/mol. Finally, calculate the mEq. Using the formula above, the milliequivalent is calculated to be: mEq = mg * V / MW. mEq = 25*3/40. mEq = 1.875. Enter the milligrams (mg) of substance, valence, and molecular weight into the calculator to determine the mEq.

(PPTX) LARUTAN PEKAT DOKUMEN.TIPS
Calcium (Ca) 40. 2. Note: The milliequivalent (mEq) is the unit of measure often used for electrolytes. It indicates the chemical activity, or combining power, of an element relative to the activity of 1 mg of hydrogen. Thus, 1 mEq is represented by 1 mg of hydrogen (1 mole) or 23 mg of Na+, 39 mg of K+, etc. a As phosphorus, inorganic.
Convert Micrograms To Milligrams Convert mcg to mg (Micrograms to Milligrams) Converter
Sodium Chloride (NaCl) dissociates to give one sodium cation and one chloride anion. The valence is the absolute charge on either the sodium cation or the chloride anion. Hence, the valence is 1. Step 3: Plug the values in steps 1 and 2 in the mEq equation. mEq = (milligrams x valence)/molecular weight. mEq = (9000 x 1)/58.5. mEq = 153.85

1 Gram Berapa Mg Sinau
milligrams = 100 mg. atomic weight of sodium = 22.99 g/mol. Number of valence = 1. Put all values into the formula. mEq = (100mg * 1) / 22.99 = 4.3497 mEq. mEq ≈ 4.3497 mEq. 100mg of sodium is equivalent to approximately 4.3497 mEq.