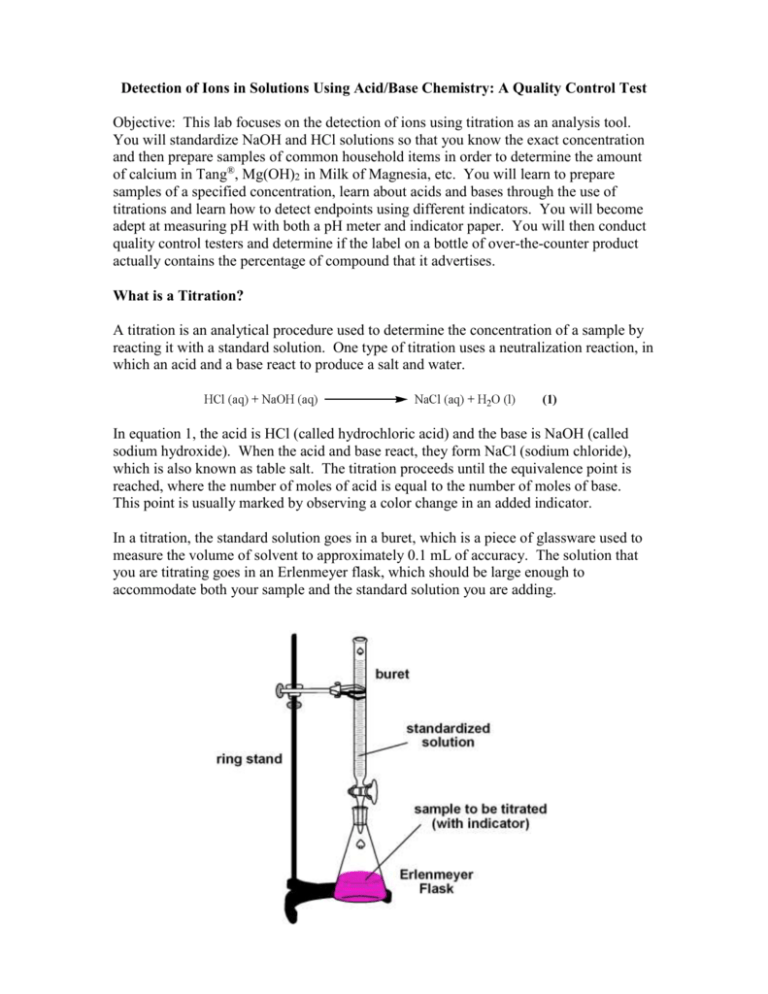
Standardizing a Sodium Hydroxide (NaOH) Solution
The concentration of NaOH in the solution is 1.0 × 10 − 3 M . Step 2: Calculate [ OH − ] based on the dissociation of NaOH Because NaOH is a strong base, it dissociates completely into its constituent ions in aqueous solution:

10 NaoH Solution YouTube
Sodium hydroxide solution min. 10% (1.11) for analysis EMSURE®; CAS Number: 1310-73-2; Synonyms: Sodium hydroxide solution; Linear Formula: NaOH; find Supelco-105588 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich

Natronlauge 10 (NaOH) 1 Liter CRIDA CHEMIE
Suppose that we now add 0.20 M NaOH to 50.0 mL of a 0.10 M solution of HCl. Because HCl is a strong acid that is completely ionized in water, the initial [H +] is 0.10 M, and the initial pH is 1.00.Adding NaOH decreases the concentration of H + because of the neutralization reaction: OH − + H + ⇌ H 2 O (in part (a) in Figure 16.5.2).Thus the pH of the solution increases gradually.

10N Sodium Hydroxide, For Laboratory, Grade Standard Technical Grade at Rs 1440/bag in Pune
NaOH (Sodium hydroxide) The preparation of 10 N NaOH involves a highly exothermic reaction, which can cause breakage of glass containers. Prepare this solution with extreme care in plastic beakers. To 800 mL of H 2 O, slowly add 400 g of NaOH pellets, stirring continuously. As an added precaution, place the beaker on ice.
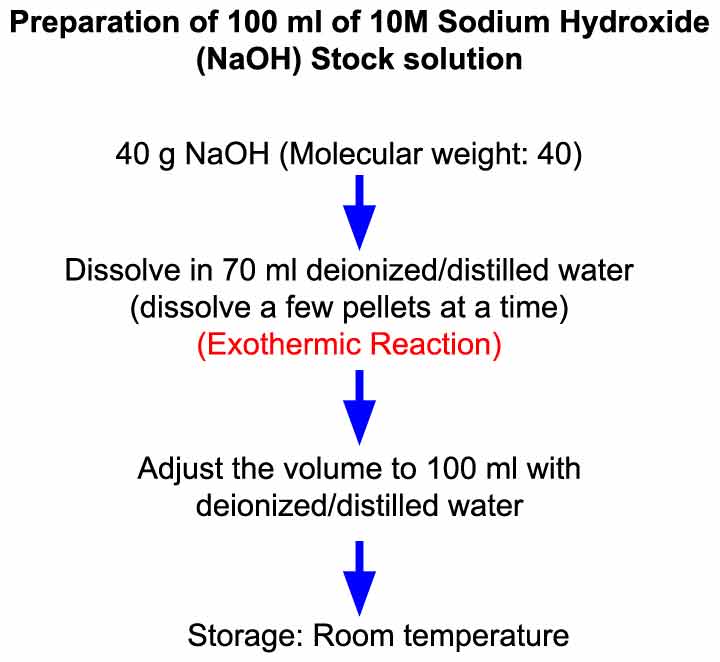
Preparation of 10 M Sodium Hydroxide (NaOH) Solution Laboratory Notes
Sodium hydroxide. Molecular Formula HNaO. Average mass 39.997 Da. Monoisotopic mass 39.992512 Da. ChemSpider ID 14114. - Charge.

A Amostra De 100 Ml De Naoh EDUCA
Use personal protective equipment (lab coat, gloves, goggles etc) for your safety and follow the guidelines of your institute. Step 1: To prepare 100 ml of 10 M NaOH solution, weigh out 40 g of NaOH (molecular weight: 40). Precaution:Don't expose solid NaOH to air for a long time while weighing. NaOH is hygroscopic in nature and absorbs water.
/Sodium-Hydroxide-58e67c825f9b58ef7ec9a02e.jpg)
How to Prepare a Sodium Hydroxide or NaOH Solution
SODIUM HYDROXIDE SOLUTIONS (MORE THAN 10% NaOH) 1. Product Identification. Synonyms: Caustic soda solution; lye solution; sodium hydroxide liquid; sodium hydrate solution,Sodium Hydroxide Concentrate Solution StandARd®, Sodium Hydroxide, DILUT-IT® Analytical Concentrates, sodium hydroxide volumetric solutions CAS No.: 1310-73-2.

NaOH Chemical Name Sodium Hydroxide Common, Compound Name
Stir the sodium hydroxide, a little at a time, into a large volume of water and then dilute the solution to make one liter. Add sodium hydroxide to water— do not add water to solid sodium hydroxide. Be sure to use borosilicate glass (e.g., Pyrex) and consider immersing the container in a bucket of ice to keep the heat down.
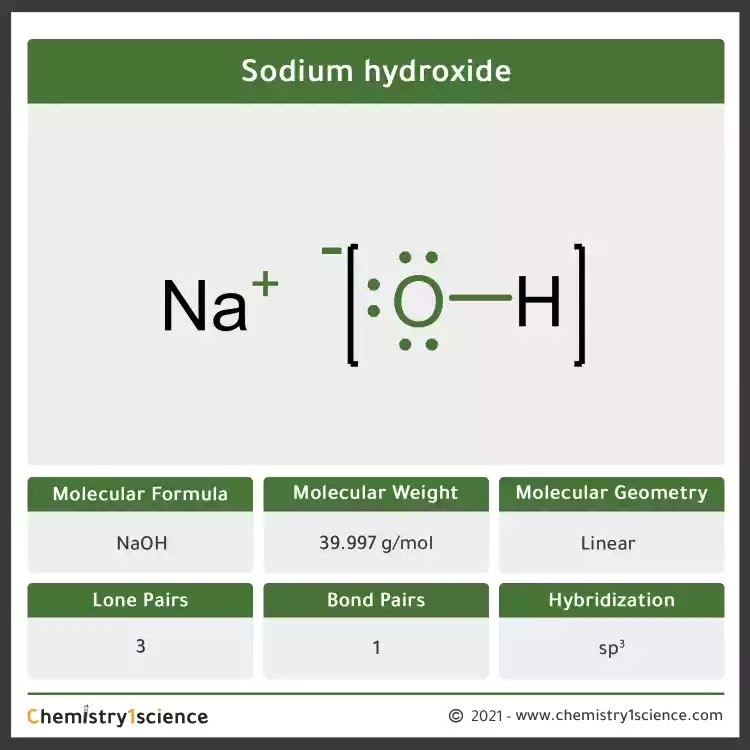
Molecular weight of naoh
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.

10M Sodium Hydroxide (NaOH) Solution TBS5052
2024-03-02. Description. At room temperature, sodium hydroxide is a white crystalline odorless solid that absorbs moisture from the air. It is a manufactured substance. When dissolved in water or neutralized with acid it liberates substantial heat, which may be sufficient to ignite combustible materials.

Naoh sodium hydroxide molecule Royalty Free Vector Image
Sodium hydroxide solution (10.0N); CAS Number: 1310-73-2; Synonyms: Sodium hydroxide solution,normality; Linear Formula: NaOH; find Supelco-SX0607N MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich
COMMON NAME FOR SODIUM HYDROXIDE
10 NaOH molecular weight. Molar mass of 10 NaOH = 49.99711 g/mol. Convert grams 10 NaOH to moles. or. moles 10 NaOH to grams. Molecular weight calculation: 10 + 22.98977 + 15.9994 + 1.00794. Percent composition by element. Element: Sodium Symbol: Na Atomic Mass: 22.989770 # of Atoms: 1 Mass Percent: 65.983%.

Chemical Formula Of Sodium Hydroxide
A 10% (weight/volume) sodium hydroxide (NaOH) stock solution can be prepared by dissolving 10 g of NaOH pellet in deionized/distilled water to a final volume of 100 ml. The detailed procedure is described below. REQUIREMENTS. Reagents and solutions Sodium hydroxide (NaOH) pallet Deionized/Double distilled water. Equipment and disposables

preparation of 10NaOH SOLUTION. YouTube
10 % NaOH means 100 ml of solution containing 10 g of NaOH. Given that, the number of NaOH solution = 10 %. The molar mass of NaOH = 40 g / mol. Thus, the number of moles 10 % NaOH = 10 40 = 1 4 = 0. 25 moles. Volume of 10 % NaOH solution = 100 1000 = 0. 1 L. Molarity(M) = 0. 25 0. 1 = 2. 5 M.
:max_bytes(150000):strip_icc()/sodium-hydroxide-58c849873df78c353c6377fc.jpg)
Names of 10 Bases With Chemical Structures and Formulas
SODIUM HYDROXIDE SOLUTION 10 MOL/L. 1.00221. SUITABLE FOR CLEANING IN PLACE. View Pricing. All Photos (3) Sodium hydroxide solution 10 mol/L. Synonym(s): Sodium hydroxide solution. Linear Formula: NaOH. CAS No.: 1310-73-2. Molecular Weight: 40.00. 4.80648.
Sodium hydroxide (NaOH) is classified as a strong base. For every mole of sodium hydroxide added
Sodium hydroxide (NaOH) - Sodium hydroxide is an ionic compound. The molecular weight of sodium hydroxide is 40 g/mol. It is a white, translucent crystalline solid and used in the manufacturing of detergents and soaps. To learn about the structure, Properties , Preparation , Uses, Health Hazards and FAQs of Sodium hydroxide (NaOH) . Visit BYJU'S for more information.