Acetanilide analgesic drug molecule (obsolete). Skeletal formula Stock Vector Image & Art Alamy
Preparation of 5,7-dimethyl-1H-indazole-3-carboximidothioic acid. A solution of the Step 4 product (22.0 mmol) dissolved in 50 ml 20% triethylamine/pyridine was cooled to 0°C and saturated with H 2 S for 5 minutes. The vessel was then sealed and the mixture stirred 90 minutes at ambient temperature.

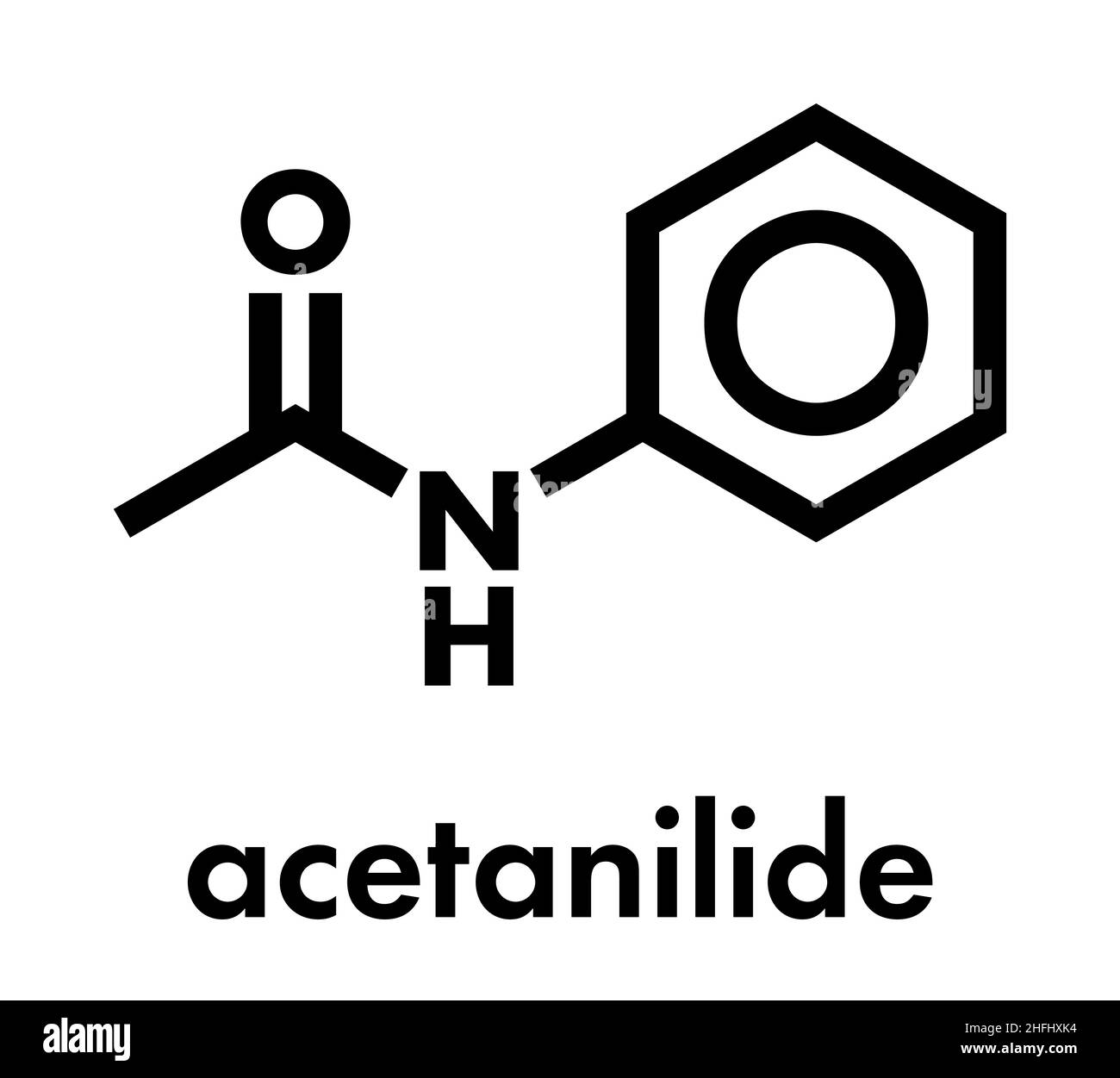
Acetanilide Chemical Structure
Acetanilide or N-phenylacetamide is an aromatic compound having phenyl ring attached to an acetamido group (-NHCOCH3). In 1886, acetanilide was introduced as an analgesic and antipyretic drug into medical practice by A. Cahn and P. Hepp. Since then, many acetanilide derivatives have been found to have antimicrobial, analgesic, anti-inflammatory, antipyretic, antioxidant, anticonvulsant, anti.

Acétanilide Définition et Explications
Acetanilide. Molecular Formula CHNO. Average mass 135.163 Da. Monoisotopic mass 135.068420 Da. ChemSpider ID 880.

Lab4 Acetanilide LAB 4 Synthesis of Acetanilide Introduction Make sure to include a
The Henry's Law constant for acetanilide is estimated as 6.2X10-9 atm-cu m/mole(SRC) using a fragment constant estimation method(1). This value indicates that acetanilide will be essentially nonvolatile from water surfaces(2,SRC). Acetanilide's Henry's Law constant(1,SRC) indicates that volatilization from moist soil surfaces may not occur(SRC).

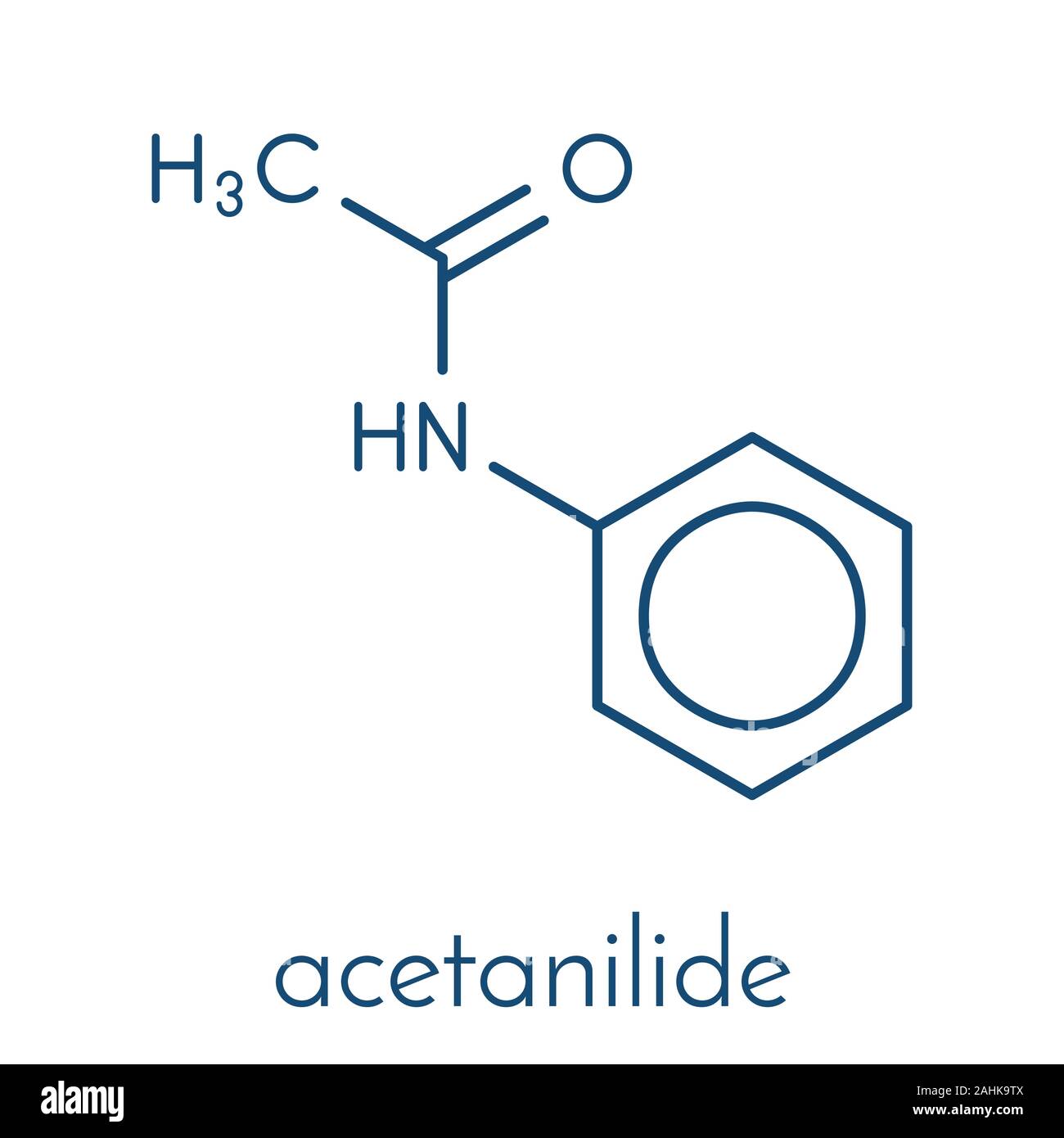
Acetanilide Chemical Structure
acetanilide, synthetic organic compound introduced in therapy in 1886 as a fever -reducing drug. Its effectiveness in relieving pain was discovered soon thereafter, and it was used as an alternative to aspirin for many years in treating such common complaints as headache, menstrual cramps, and rheumatism. Excessive or prolonged use engenders.
Me acetanilid hires stock photography and images Alamy
Acetanilide undergoes palladium-catalyzed cross-coupling reaction to form ortho-acylacetanilide. Application. Acetanilide is used as a EOF (electroosmotic flow) marker in the studies of affinity capillary electrophoresis for drug-protein binding. Safety Information. Pictograms. GHS07. Signal Word.
Drug Central
Monograph ID M1319 Title Acetanilide UNII SP86R356CC Molecular formula C 8 H 9 NO Molecular weight 135.17 Percent composition C 71.09%, H 6.71%, N 10.36%, O 11.84%
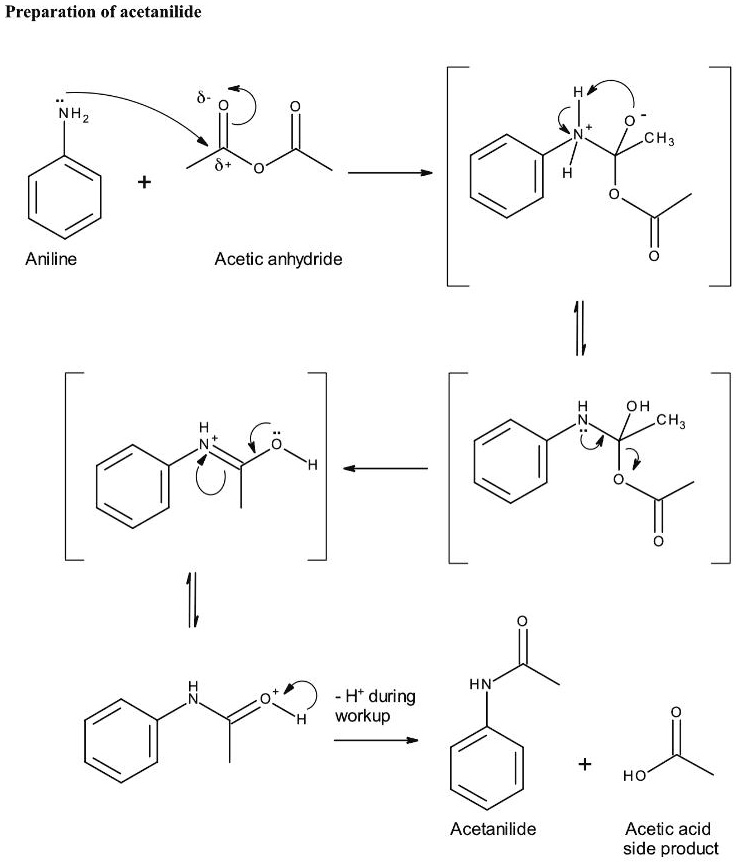
SOLVED Preparation of acetanilide NHz CH Aniline Acetic anhydride HN Ht 'during workup HO
Acetanilide was the first aniline derivative serendipitously found to possess analgesic as well as antipyretic property. The literature review shows that the synthesis and characterization of.

acetanilide cas 103844 Haihang Industry Co., Ltd.
Acetanilide is a synthetic organic compound introduced clinically in 1886 as a fever-reducing drug. Its effectiveness in relieving pain was discovered soon thereafter and it was used as an alternative to aspirin for many years in treating such common complaints as headaches, menstrual cramps, and rheumatism.

Preparation acetanilide aniline From aniline in the Laboratory
Preparation of acetanilide by the acetylation of aniline is an important exercise in organic synthesis at the undergraduate level. It involves treating aniline with an acetylating agent in the presence of an acid, base, or reducing agent and very often requires heating. Here, we report a safe and green procedure for this synthesis. This method is not only very convenient but also gives an.

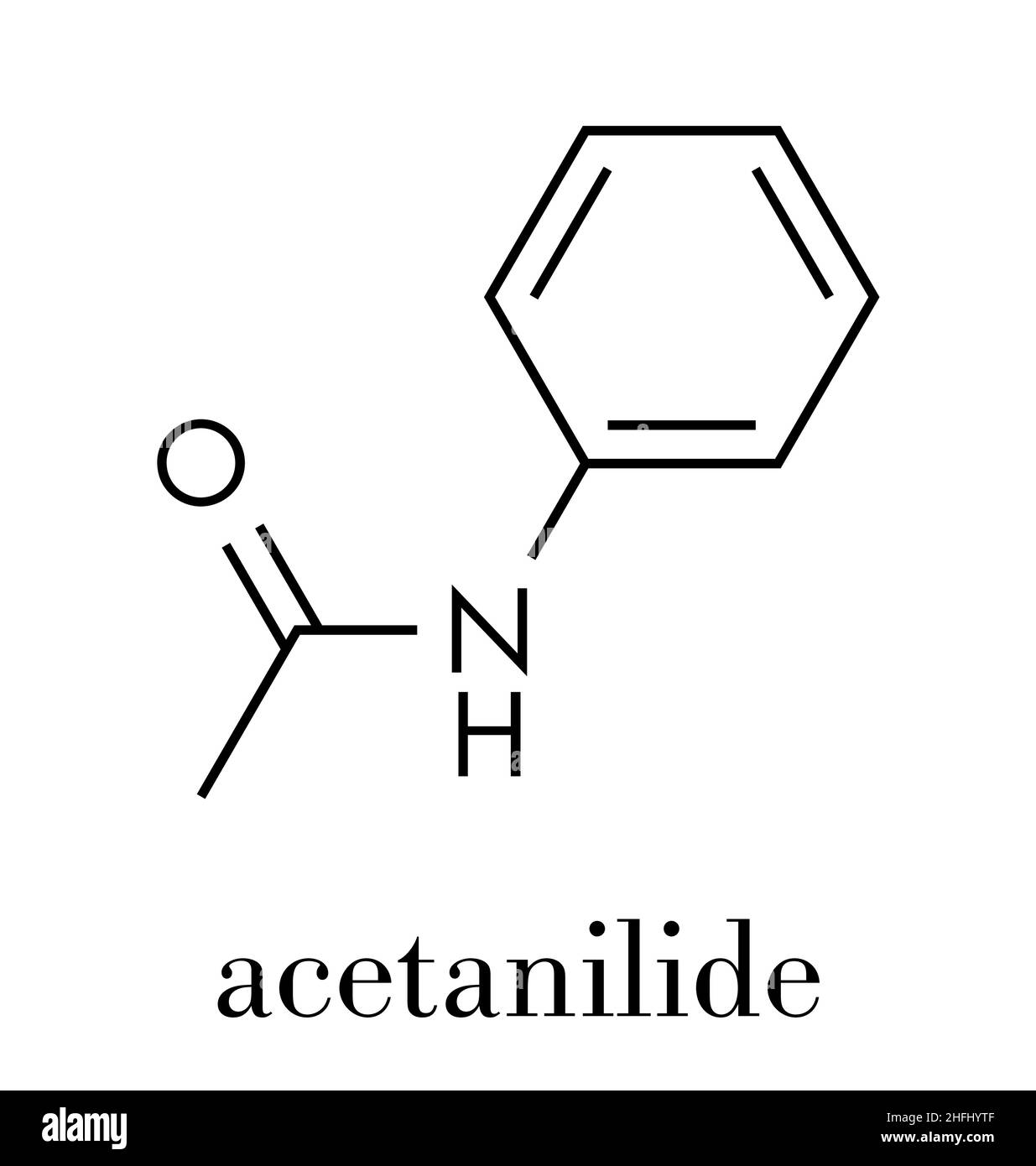
Acetanilide Chemical Structure
Acetanilide is mainly used as an intermediates for the synthesis of pharmaceuticals and as an additive in hydrogen peroxide, varnishes, polymers and rubber.The most probable human exposure would be occupational exposure through dermal contact or inhalation at workplaces where acetanilide is produced or used.
Paracetamol analgesic drug molecule Black and White Stock Photos & Images Alamy
Acetanilide is a derivative of aniline, where one of the hydrogens on the nitrogen atom has been replaced with an acetyl group. Acetanilide has a wide variety of uses and is a useful building.

Acetanilide, 99+, Thermo Scientific Chemicals Fisher Scientific
Robert Chênevert. The acid hydrolysis of cis and trans methoxy bicyclic and tricyclic orthoesters 1-4 was studied. The trans isomers gave only the corresponding hydroxy-lactones (2 → 14 and 4.

Acetanilide Chemical Structure
Acetanilide is an odourless solid chemical of leaf or flake-like appearance. It is also known as N-phenylacetamide, acetanil, or acetanilid, and was formerly known by the trade name Antifebrin. Preparation and properties. Acetanilide can be produced by reacting acetic anhydride with aniline: C 6 H 5 NH.
Structural Formula Of Acetanilide
IUPAC Standard InChIKey: FZERHIULMFGESH-UHFFFAOYSA-N Copy CAS Registry Number: 103-84-4 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Other names: Acetanilide; Acetamidobenzene; Acetanil; Acetoanilide; Acetylaniline; Antifebrin; Benzenamine, N-acetyl-; N-Acetylaniline; N-Phenylacetamide.
Acetanilide Chemical Structure
ACETANILIDE is an amide. Flammable gases are formed by the reaction of organic amides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates.