
In the equation Cr2O72 + Fe2+ + H+ Balacing Redox Master Series Balancing equation trick
Ammonium dichromate is an inorganic compound with the formula (NH 4) 2 Cr 2 O 7.In this compound, as in all chromates and dichromates, chromium is in a +6 oxidation state, commonly known as hexavalent chromium.It is a salt consisting of ammonium ions and dichromate ions.. Ammonium dichromate is sometimes known as Vesuvian Fire, because of its use in demonstrations of tabletop "volcanoes".

Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5, Cr2O72 and NO3
1. Introduction. As one of the most widely used metal ions in modern industry and agriculture, chromium, especially hexavalent chromium (Cr(VI)), is always stable in ecosystems [1, 2].However, Cr(VI) is highly toxic, easy to bioaccumulate and nonbiodegradable in water environment, and poses a significant risk to humans and most animals because it may cause hereditary genetic defects, allergic.
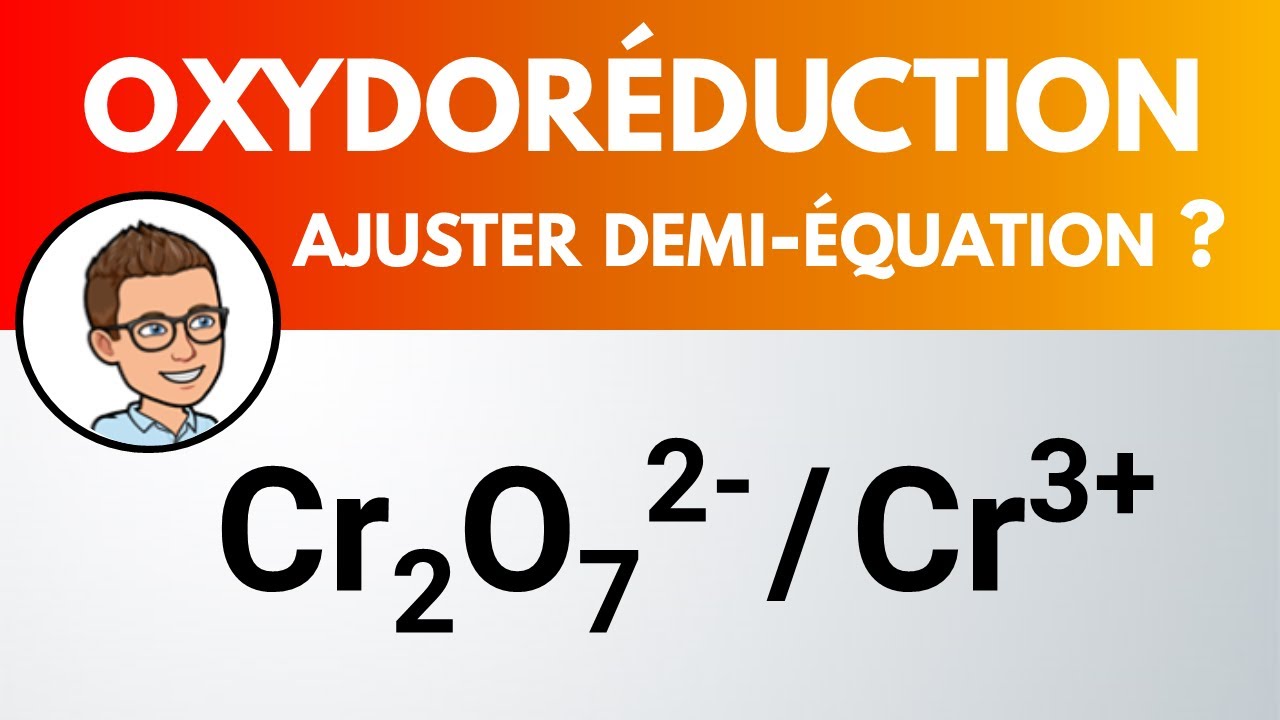
Demiéquation redox Cr2O72 / Cr3+ PhysiqueChimie YouTube
Cr 2 O 7 2-(dichromate) has two chromium atoms and seven oxygen atoms.. In the Cr 2 O 7 2-Lewis structure, there are two single bonds around the center oxygen atom, with two chromium atoms attached to it, and each chromium makes two single bonds and two double bonds with other three oxygen atoms. The oxygen atom with single bonds has three lone pairs, the oxygen atom with double bonds has two.
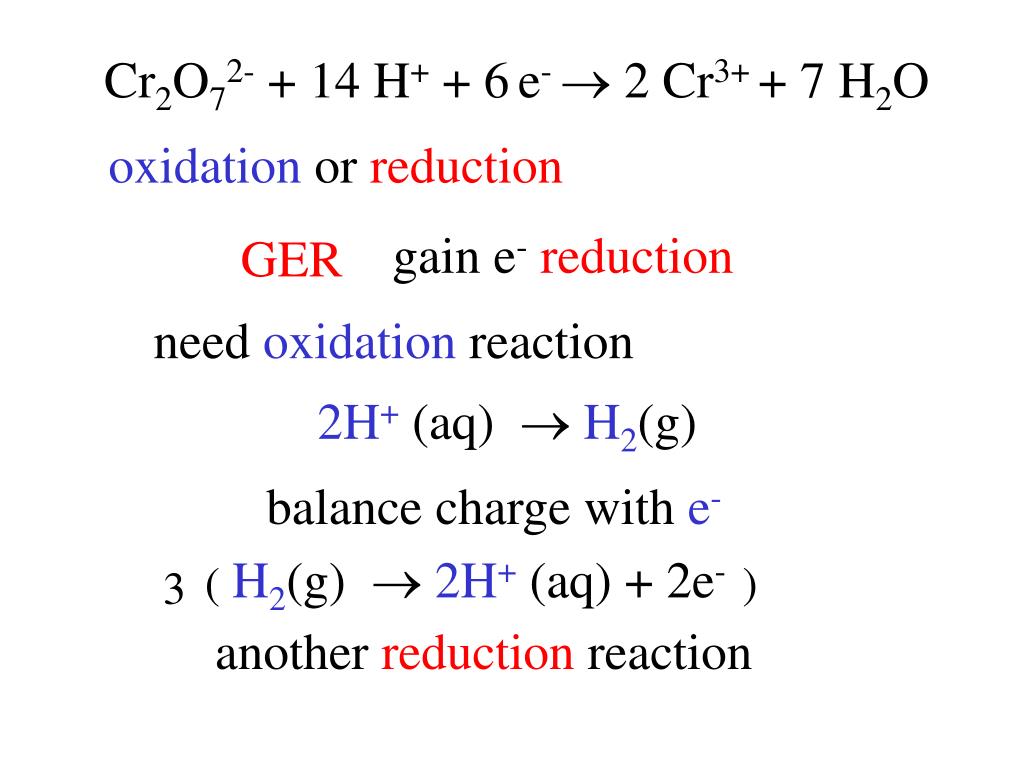
PPT Electrochemistry PowerPoint Presentation, free download ID6595241
Structure, properties, spectra, suppliers and links for: dichromate, 13907-47-6.

Solved For the following reaction in acidic solution Cr2O72+
Potassium dichromate is an orange crystalline solid that gives chromic oxide and potassium chromate when it readily decomposes. Dichromate's molecular formula is Cr 2 O 72- and its density is 2.68 grams per centimetre cube. The molecular weight weighs 294.185 g/mol over molar mass. This compound also has a boiling point of 500 C and it also.

Cr2O72+HNO2=Cr3++NO3 balance the chemical equation mydocumentary838. YouTube
Explore the structural formula and geometry of the dichromate ion, a powerful oxidizing agent that forms bright orange crystals, with Purdue University.

Consider the reaction Cr2O72 + 14H+ + 6e → 2Cr3+ + 7H2OWhat is the quantity of electricity
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 427 CH3OH + 6 Cr2O72- + 10 H+ = 427 CH2O + 4 Cr3+ + 432 H2O. Reactants. Products.

balance Cr2O72 + Fe3+ to give Cr3+ + Fe3+ in acidic Medium YouTube
Cucurbit[6]uril (Q[6]) could serve as a selective absorbent for the toxic anion Cr2O72-, which was demonstrated by the results of UV-vis, ICP, XPS, SEM, and EDS experiments. Single-crystal X-ray diffraction analysis revealed that capture capacity could be attributed to the outer-surface interactions of cucur

PPT Chapter 21 PowerPoint Presentation, free download ID5404965
In this video, I will talk about how to write a Redox Half Equation using Dichromate ion to Chromium 3+ using the 5 Steps 1) Balanced atom undergoing reducti.
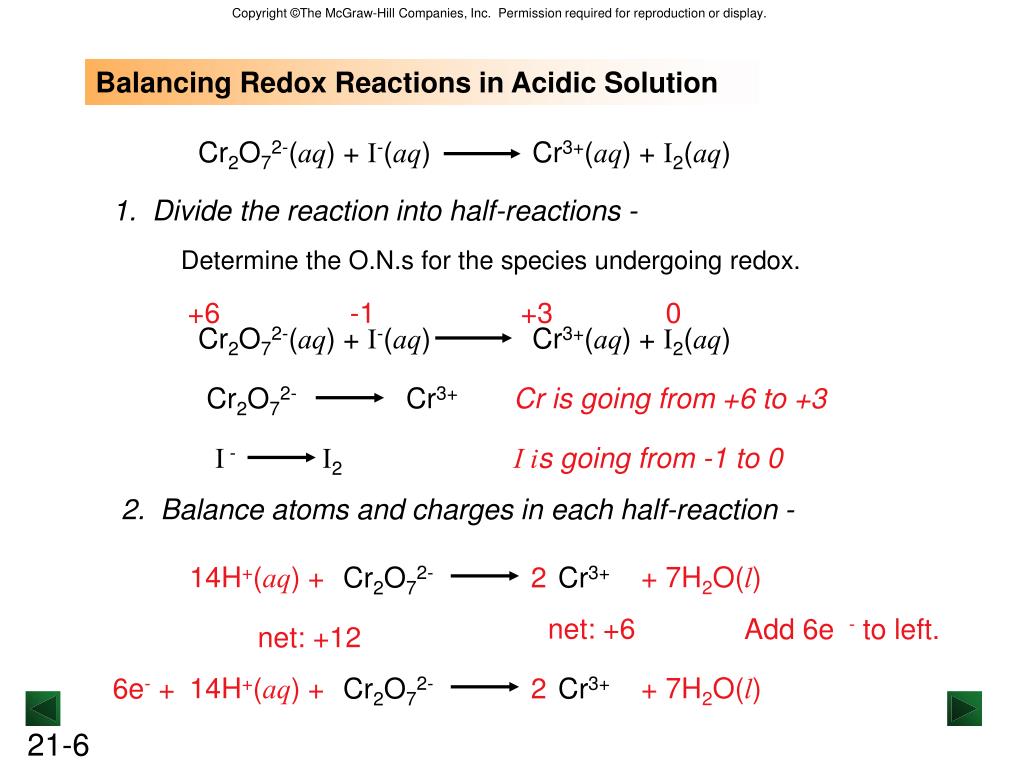
PPT Chapter 21 PowerPoint Presentation, free download ID5404965
For the dichromate ion, Cr 2 O 72- the element with a standard oxidation state is oxygen which is -2, and we need to determine the oxidation state of the chromium. Rule 8 - The oxidation of oxygen is -2: Assign x for the oxidation state of Cr and set up an equation. Remember, for ions, the sum of the oxidation numbers multiplied by their.

In the dichromate dianion Chemistry Questions
Dichromate is an anion with the chemical formula Cr2O72-. It is used as a strong oxidising agent in organic chemistry as well as a primary standard solution in volumetric analysis. The chromate ion and dichromate ions are interconvertible in aqueous solution. The most common compound known is potassium dichromate which is an orange crystalline.

calculate the oxidation number in Cr2O72 ion on chromium (cr) atom Brainly.in
x = 12. So, two chromium atoms have a total oxidation number of +12. Since one is not more electronegative than the other, we can divide the number by two to get one chromium's oxidation number. 12 2 = 6. So, a chromium atom here has an oxidation number of +6. Answer link. +6 This is the dichromate ion, Cr_2O_7^ (2-).

Assertion In Cr2O7^(2) ion, all the CrO bond lengths are equal. Reason In Cr2O7^(2) ion all
The response surface methodology was employed to synthesize one hydrogel envisaging the optimum balance between swelling and stability for the superadsorption of Cu(II), Cd(II), Pb(II), Cr2O72−.

The established structure model of (a) Cr2O72, (b) Top view of... Download Scientific Diagram
Chromate salts contain the chromate anion, CrO2−. 4. Dichromate salts contain the dichromate anion, Cr. 2O2−. 7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.
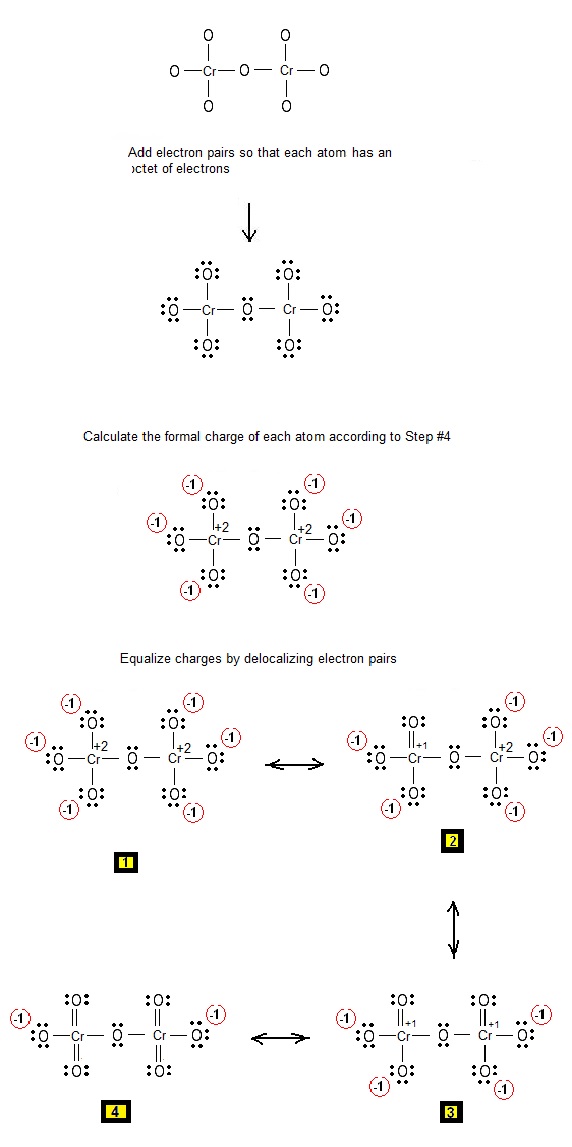
Lewis Electron Dot Structure of dichromate Cr2O72 Chemistry Net
O. 7. 2-. ) Lewis Structure. Lewis structure of dichromate (Cr 2 O 72-) ion is drawn step by step in this tutorial. Total valence electrons of two chromium atoms, seven oxygen atoms and two negative charges are considered to draw the Cr 2 O 72- Lewis structure. There are double bonds and single bonds in the lewis structure of Cr 2 O 72- ion.
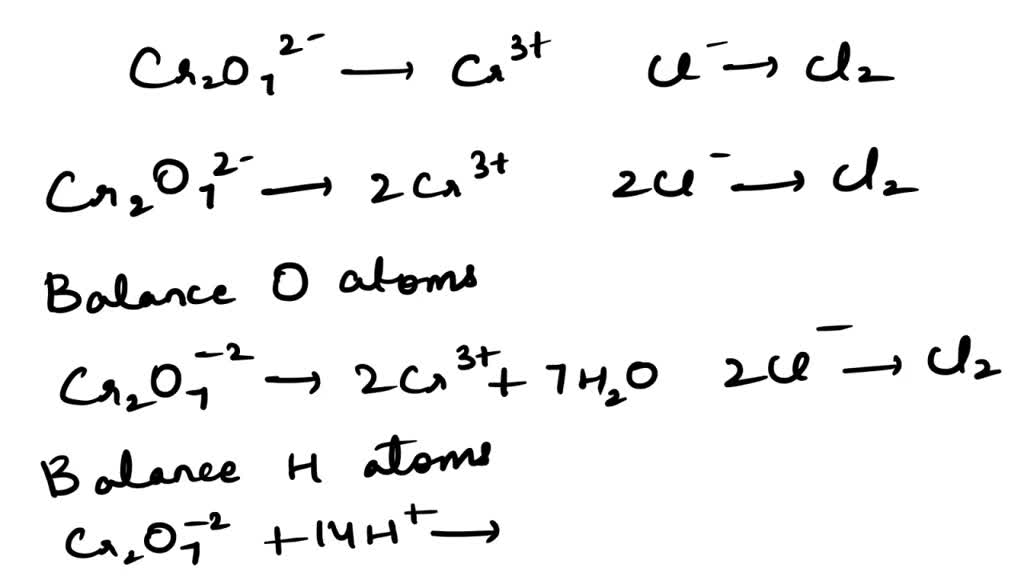
SOLVED Balance the following equation of Oxidationreduction reaction in basic conditions
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. -1 Cr2O72- + 5 H2O2 + -8 H+ = -2 Cr3+ + O2 + H2O. Reactants.