
which of the following compounds is (are) hydrolyzed tobutanoic acid upon heating in H20, H2SO4
The ethyl butanoate molecule contains a total of 19 bond (s). There are 7 non-H bond (s), 1 multiple bond (s), 4 rotatable bond (s), 1 double bond (s), and 1 ester (s) (aliphatic). Images of the chemical structure of ethyl butanoate are given below: The 2D chemical structure image of ethyl butanoate is also called skeletal formula, which is the.
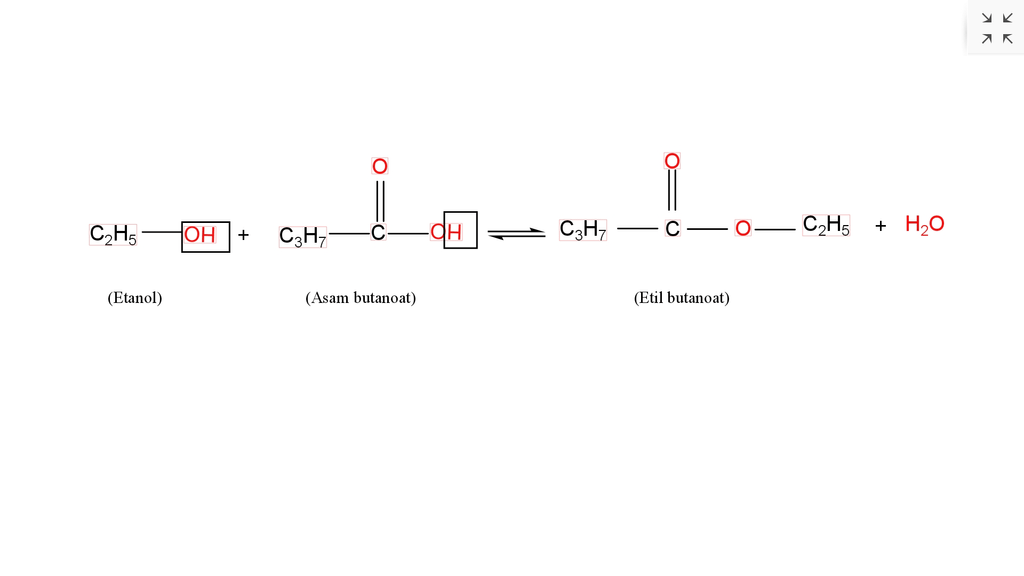
Hasil reaksi dari. A. propil butanoat B. e...
119-120 °C Alfa Aesar: 121 °C Food and Agriculture Organization of the United Nations Ethyl butanoate: 121 °C OU Chemical Safety Data (No longer updated) More details: 119-120 °C (Literature) Alfa Aesar L06025 119.9 °C FooDB FDB012082: 120 °C Sigma-Aldrich SIAL-75563

Be sure to answer all parts. ethyl butanoate, ch3ch2ch2co2ch2ch3, is one of the many organic
Keyword:'ethyl butanoate' Showing 1-30 of 75 results for "ethyl butanoate" within Products. Products Building Blocks Explorer Genes Papers Technical Documents Site Content Chromatograms. Filter & Sort. All Photos (1) Ethyl 4-(dimethylamino)butanoate. Linear Formula: C 8 H 17 NO 2. CAS No.: 22041-23-2. PH002341.

Asam 2 Etil Butanoat Meteor
Ethyl Butanoate, CH 3 CH 2 O 2 CCH 2 CH 2 CH 3. The following structure represents the ester called ethyl butanoate, CH 3 CH 2 O 2 CCH 2 CH 2 CH 3. Click here to see instructions for manipulating this structure. Click here to return to the Ester webpage. State at room T and P - liquid.

Ethyl nbutanoate CAS 105544 Odour Threshold Value
In basic hydrolysis, the molecule of the base splits the ester linkage. The acid portion of the ester ends up as the salt of the acid (in this case, the potassium salt). The alcohol portion of the ester ends up as the free alcohol. Exercise 11.9.2 11.9. 2. Write the equation for the hydrolysis of ethyl propanoate in a sodium hydroxide solution.

Ethyl 2methylbutyrate, 98, Thermo Scientific Chemicals, Quantity 100 mL Fisher Scientific
Notice: Except where noted, spectra from this collection were measured on dispersive instruments, often in carefully selected solvents, and hence may differ in detail from measurements on FTIR instruments or in other chemical environments. More information on the manner in which spectra in this collection were collected can be found here. Notice: Concentration information is not available for.
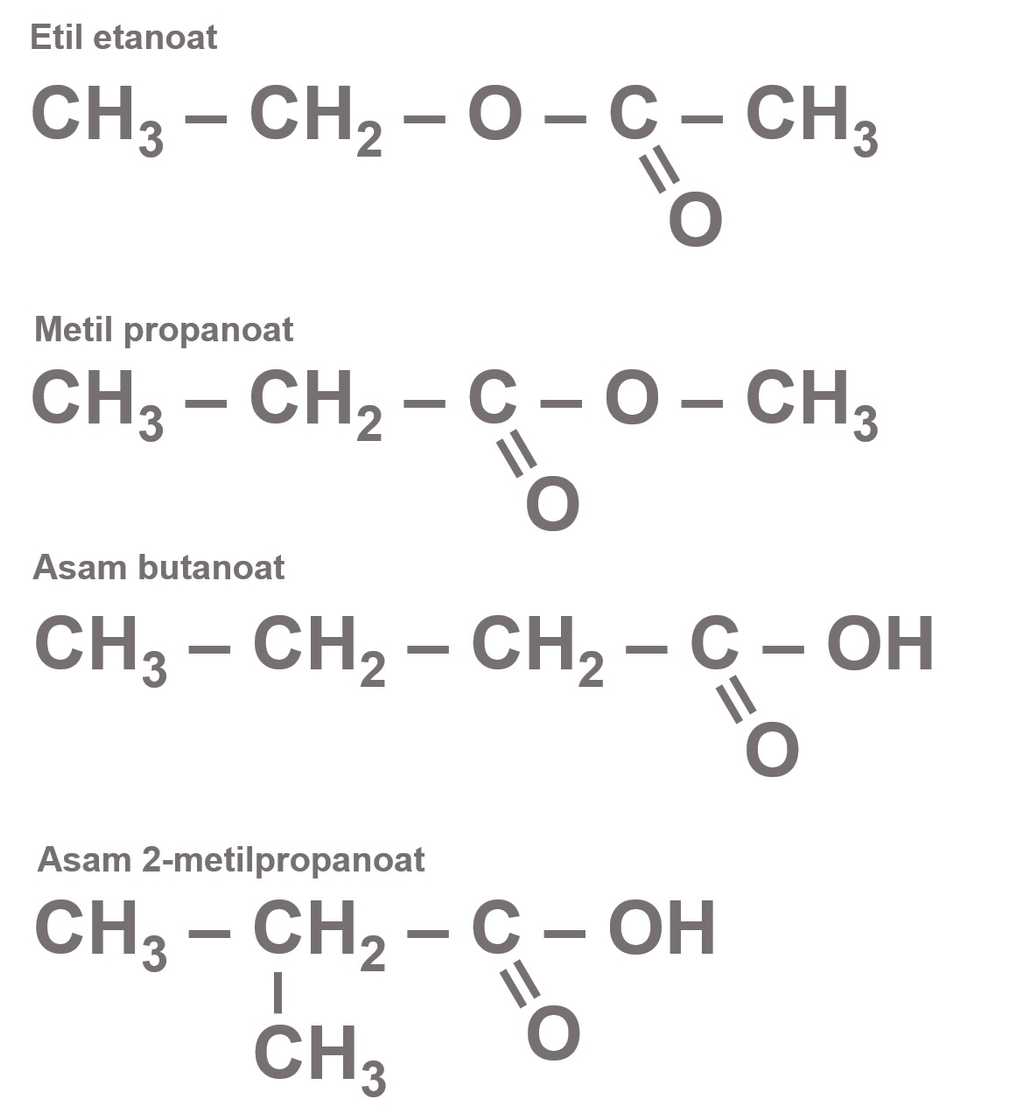
isomer C4H8O2...
Butyric acid (/ ˈ b j uː t ɪ r ɪ k /; from Ancient Greek: βούτῡρον, meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula CH 3 CH 2 CH 2 CO 2 H.It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid (2-methylpropanoic acid) is an isomer. Salts and esters of butyric acid are known.

The correct structural formula of butanoic acid is
Figure 15.5.1 15.5. 1 shows models for two common esters. Figure 15.5.1 15.5. 1: The Structure of Esters. Esters feature a carbon-to-oxygen double bond that is also singly bonded to a second oxygen atom, which is then joined to an alkyl or an aryl group. The esters shown here are ethyl acetate (a) and methyl butyrate (b).
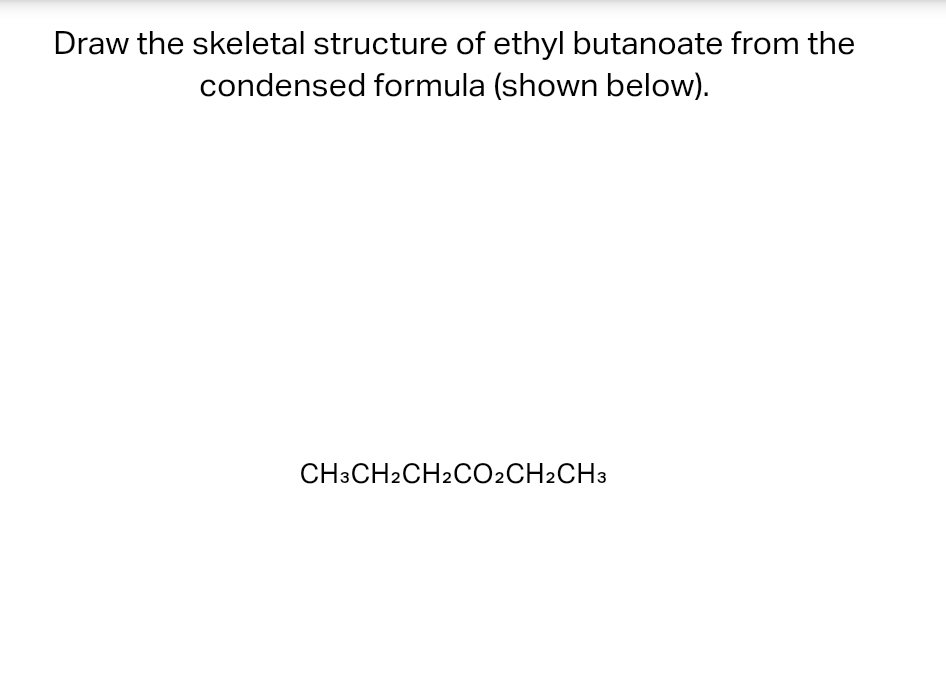
Solved Draw the skeletal structure of ethyl butanoate from
An alkyl group (in green) is attached directly to the oxygen atom by its middle carbon atom; it is an isopropyl group. The part derived from the acid (that is, the benzene ring and the carbonyl group, in red) is benzoate. The ester is therefore isopropyl benzoate (both the common name and the IUPAC name). Exercise 9.8.1 9.8. 1.
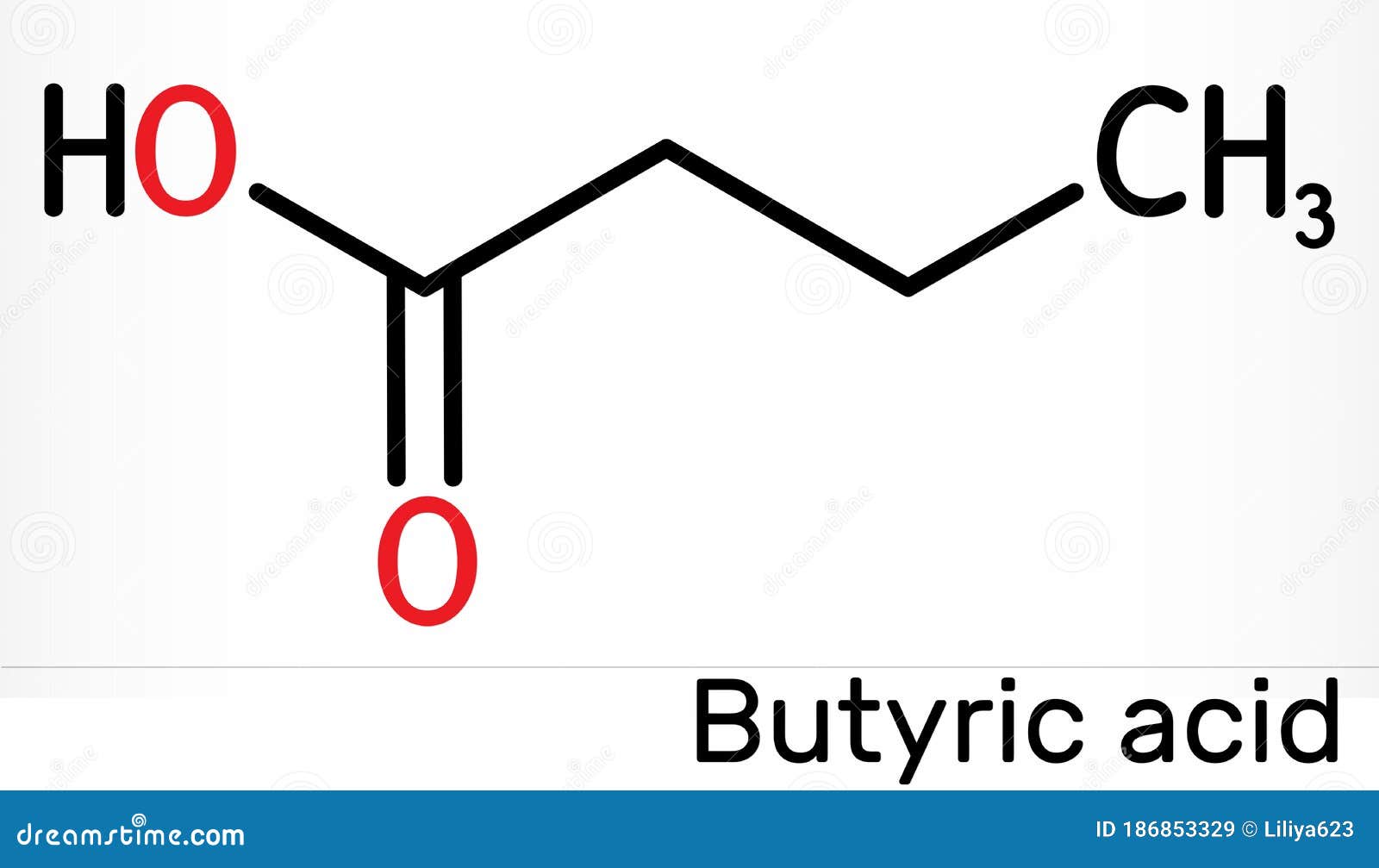
Butyric Acid, Butanoic Acid, Chemical Formula And Skeletal Structure Cartoon Vector
2-Ethylbutanoate | C6H11O2- | CID 6950112 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.

Butanoic acid, 2ethyl2methyl, 2methylpropyl ester SIELC Technologies
Ethyl butyrate, also known as ethyl butanoate, or butyric ether, is an ester with the chemical formula CH 3 CH 2 CH 2 COOCH 2 CH 3.It is soluble in propylene glycol, paraffin oil, and kerosene.It has a fruity odor, similar to pineapple, and is a key ingredient used as a flavor enhancer in processed orange juices. It also occurs naturally in many fruits, albeit at lower concentrations.
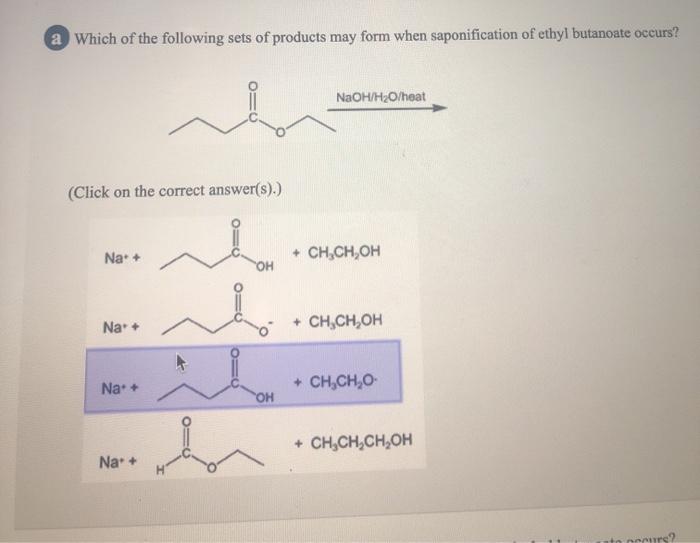
Solved a Which of the following sets of products may form
Ethyl butanoate, Butyric acid ethyl ester. Linear Formula: CH 3 CH 2 CH 2 C(O)OC 2 H 5. CAS Number: 105-54-4. Molecular Weight: 116.16. Beilstein: 506331. EC Number: 203-306-4. MDL number: MFCD00009394. PubChem Substance ID: 24894421. NACRES: NA.22. Pricing and availability is not currently available. Recommended Products. Slide 1 of 10. 1 of 10.
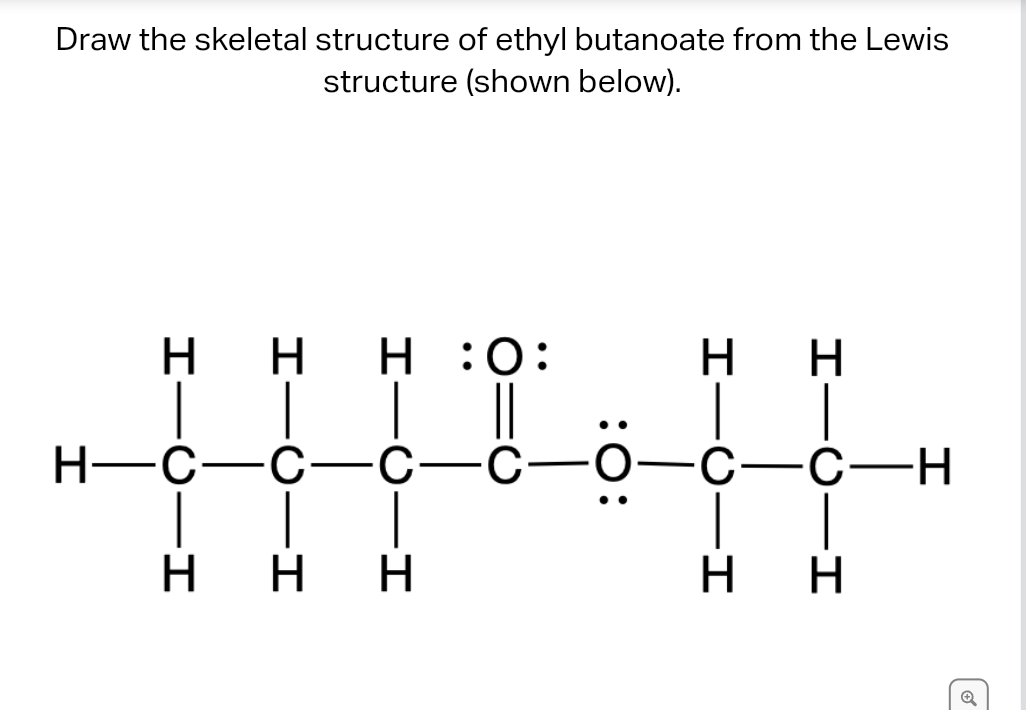
Solved Draw the skeletal structure of ethyl butanoate from
IUPAC Standard InChIKey: OBNCKNCVKJNDBV-UHFFFAOYSA-N Copy CAS Registry Number: 105-54-4 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Isotopologues:

Alfa Aesar 2Ethyl1butanol, 99 Fisher Scientific
ethyl butanoate-d3; Other names: Butyric acid, ethyl ester; Ethyl butanoate; Ethyl butyrate; Ethyl n-butyrate; Ethyl n-butanoate; n-Butyric acid ethyl ester; UN 1180; Ethyl ester of butanoic acid; NSC 8857; ethyl butanoate (ethyl butyrate); ethyl 1-butyrate Permanent link for this species. Use this link for bookmarking this species for future.
[Solved] What is the structure for ethyl butanoate? Course Hero
Ethyl butyrate is a butyrate ester resulting from the formal condensation of the hydroxy group of ethanol with the carboxy group of butyric acid. It has a role as a plant metabolite. It is functionally related to an ethanol. ChEBI.

Draw the major condensation product obtained by treatment of ethyl butanoate with sodium
Ethyl butyrate, also known as ethyl butanoate, is an organic compound that is commonly used in a wide range of applications, due to its fruity aroma and pleasant sweet taste. Its chemical formula is C 6 H 12 O 2. Physical and Chemical Properties. Ethyl butyrate is a colorless liquid that is less dense than water. It has a boiling point of 120.