
Difference Between Isotopes And Isobars Understanding The Key Differences A Plus Topper
The terms isotopes, isobars, and isotones are used to describe the interactions between the atoms of various chemical elements. The concept of the nucleus was discovered by Rutheford in his atomic model popularly known as Rutherford's atomic model, which states that ' Protons and neutrons, which constitute almost all of the mass of the.
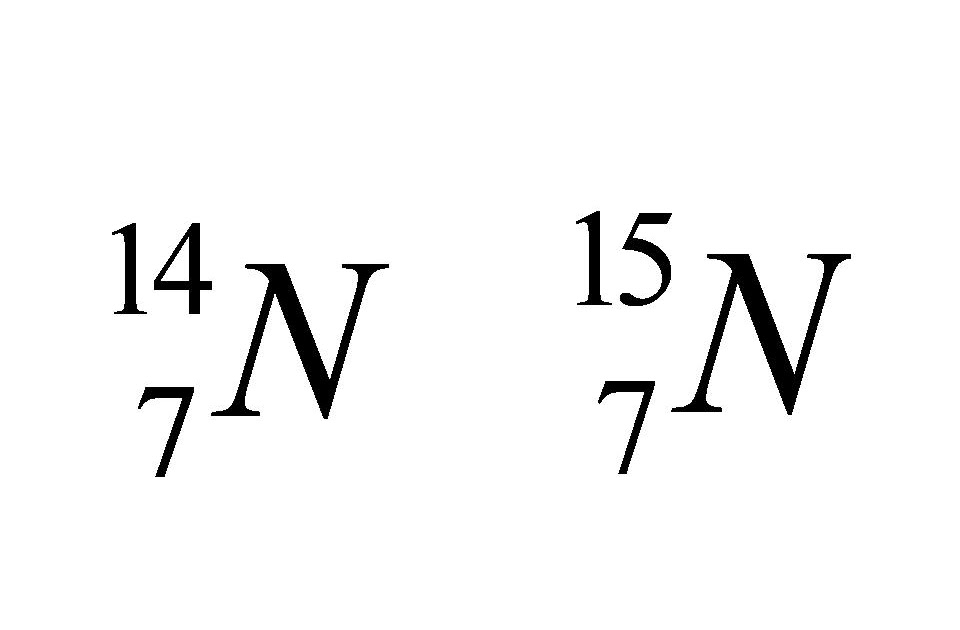
Isotop, Isoton, Isobar dan Isoelektron Chemistry is fun
Isotop, isoton, isobar, dan isoelektron merupakan salah satu materi kimia yang cukup menarik untuk dibahas. Kalau kebetulan kamu ingin belajar tentang materi ini lebih dalam, simak penjelasan lengkapnya berikut. Kami juga telah menyediakan soal latihan yang bisa dikerjakan untuk mengasah kemampuanmu.
Teori Struktur Atom Menurut Para Ahli Beserta Pengertiannya (Lengkap)
Apa perbedaan isotop, isobar dan isoton? Jawaban: Isotop. Dilansir dari Encyclopaedia Britannica, isotop adalah atom dengan nomor atom yang berbeda namun menduduki tempat yang sama pada table periodik. Jadi unsur pada table periodik yang kamu lihat sebeneranya tidak hanya satu unsur, melainkan ada dua atau lebih unsur yang duduk pada posisi.

Isotop, Isobar, dan Isoton My Chemistry ff
Isotop, Isobar, Isoton: Pengertian dan Contohnya. Berdasarkan nomor atom dan nomor massanya, atom-atom dari unsur yang sama ataupun berbeda dapat dibedakan menjadi isotop, isobar, dan isoton. Seperti diketahui, dalam ilmu kimia, kita diperkenalkan dengan konsep atom yaitu bagian terkecil dari suatu materi. Partikel dasar penyusun atom adalah.
Difference Between Isotopes and Isobars Definition, Properties, Examples
- Isobars are atoms that have the same mass number but different atomic numbers are called isobars. - The word isobar meaning 'equally heavy' is taken from the Greek isos = equal, and barys = heavy. Examples of isobars - For example, 40 Ar 18, 40 K 19, and 40 Ca 20 are isobaric atoms. - Similarly, 235 U 92, 235 Np 93, 235 Pu 94 are.

Perbedaan Isotop, Isobar, Isoton, dan Isomer Muhyidin, SKM
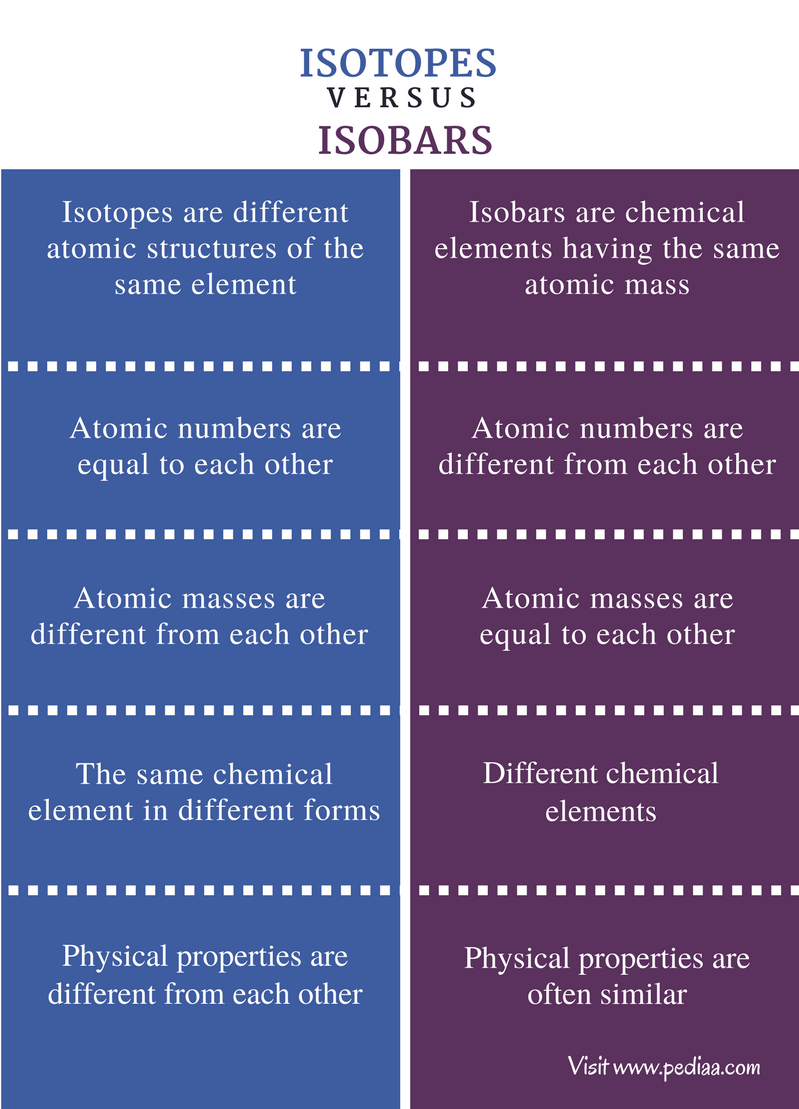
Thus, the major difference between Isotopes and Isobars is that isotopes belong to the same element while isotones are different elements. Also, isotopes have similar chemical properties. However, isobars have different chemical properties. For example, 12 C, 13 C, and 14 C are the isotopes of Carbon while 26 Fe 58 and 27 Ni 58 are Isotones.

Kimia kelas x ISOTOP ISOBAR ISOTON YouTube
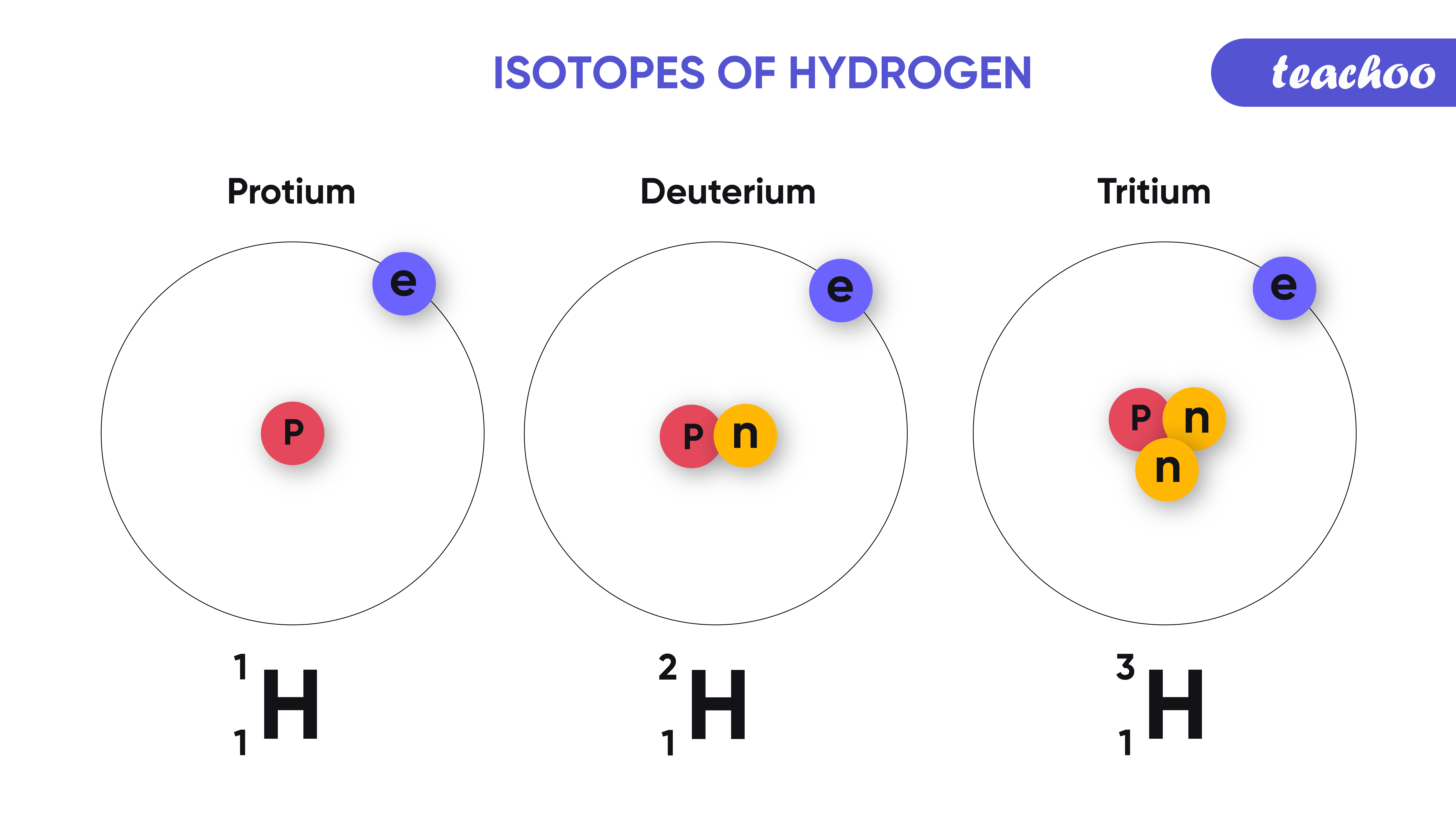
Isotopes. Isotopes are atoms of an element which have the same proton number but different nucleon numbers. Example: Hydrogen is the common example which has three isotopes. These have the same atomic number, one, but different mass numbers 1, 2, and 3. These three isotopes are commonly known as hydrogen or protium, deuterium (D) and tritium (T.
Isotopes and Isobars Definition, Uses and Difference Teachoo
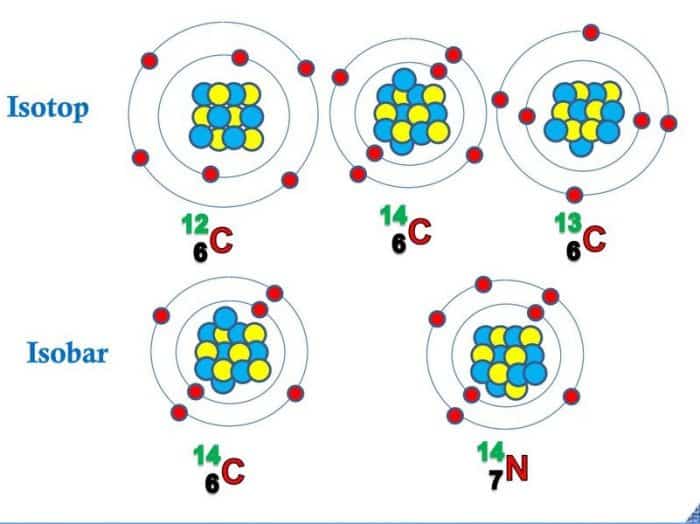
Pengertian Isobar: unsur atomnya berbeda namun memiliki nomor massa yang sama. Hal ini dinamakan isobar. Contoh Isobar. Natrium dan Magnesium dapat mempunyai nomor massa yang sama yaitu 24 Na 11 dan 24 Mg 12 Hidrogen dan Helium dapat mempunyai nomor massa yang sama yaitu 3 H 1 dan 3 He 2 Karbon dan Nitrogen dapat mempunyai nomor massa yang sama yaitu 14 C 6 dan 14 N 7. C. Isoton
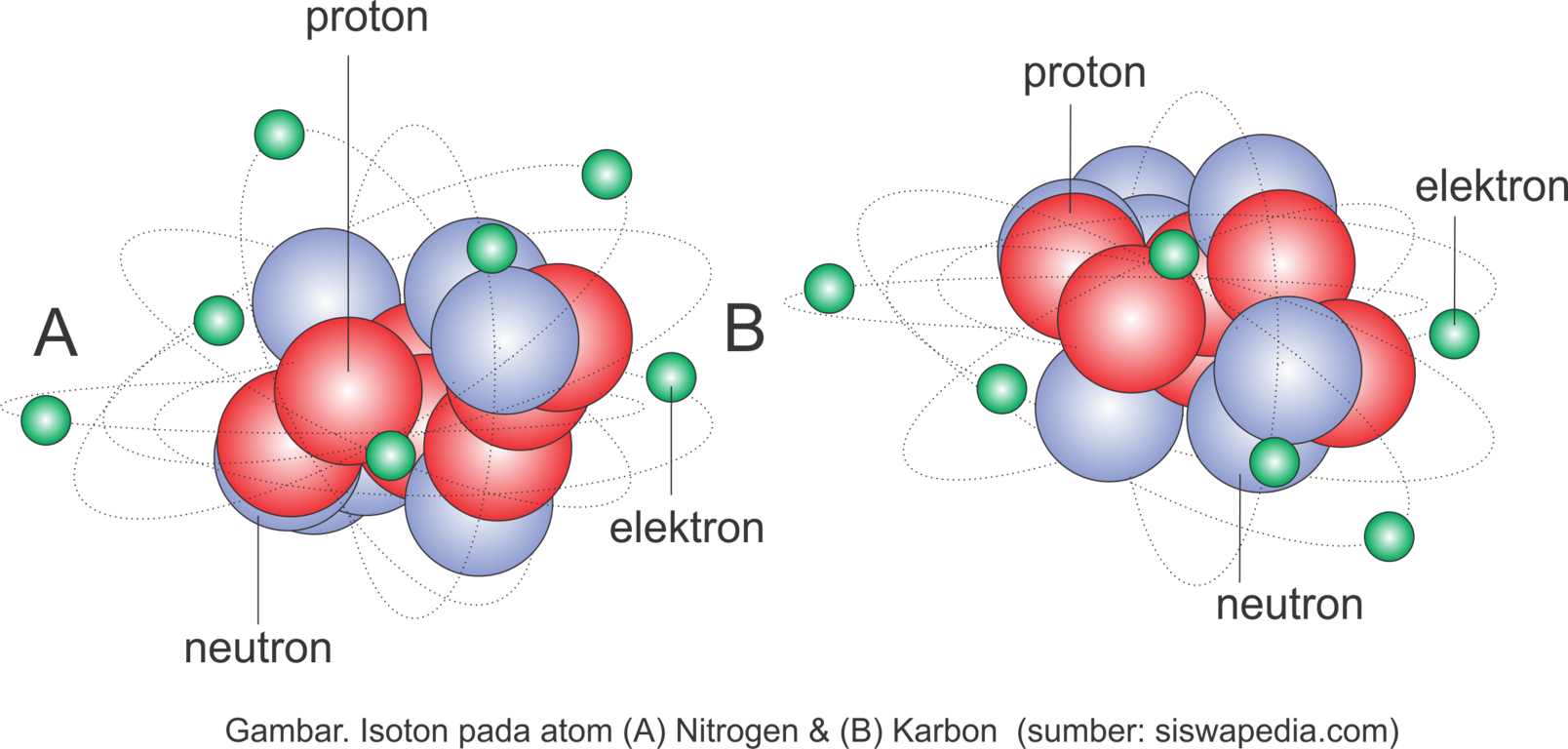
Pengertian Serta Contoh Isotop, Isobar dan Isoton Siswapedia
ISOBARS Isobar is a group of nuclides that has sum of proton and neutron are same but sum of each proton and neutron is different. • Examples : 12C6 with 12 C 7 • Examples : 12C6 with 12 C 7 5.

Chemistry Isotope, Isobar, Isotone Science Education World YouTube
Isobars. The set of elements has the same number of nucleons, where nucleons are protons or neutrons. For example, 40 Sulfur, 40 Chlorine, 40 Argon, 40 Potassium, and 40 Calcium are all isobars. Moreover, despite having the same mass number, isobars have different atomic numbers for different chemical elements.
10 Contoh Isotop Isobar Isoton dan Isoelektron Materi Kimia
As mentioned before, isotopes are atoms that have the same atomic number, but different mass numbers. Isotopes are denoted the same way as nuclides, but they are often symbolized only with the mass numbers because isotopes of the same element have the the same atomic number. Carbon, for example, has two naturally occurring isotopes, 12 6 C and.

Struktur Atom Kimia Kelas 10 • Part 3 Isotop Isobar Isoton, Massa Atom & Molekul Relatif YouTube
Isobars, Isotopes and Isotones. Isotopes are atoms having same atomic number (Protons) but different mass number. i.e., the number of neutrons are different. Hence the atomic weights of the isotopes of an element are different. Isotopes of the same element have the same chemical properties because they have the same number and arrangement of.

Isobar Isotop Isoton Studyhelp
v. t. e. Nuclide half-lives colorcoded. Two nuclides are isotones if they have the same neutron number N, but different proton number Z. For example, boron-12 and carbon-13 nuclei both contain 7 neutrons, and so are isotones. Similarly, 36 S, 37 Cl, 38 Ar, 39 K, and 40 Ca nuclei are all isotones of 20 because they all contain 20 neutrons.

10 Differences between isotopes and isobars DewWool
Iodine isobars are used to treat goiter. Cobalt isobars can be used to treat cancer. Frequently Asked Questions. Q1. What are isotopes and isobars? Answer. Isotopes are atoms of the same element with the same atomic number but a different mass number. Isobars are atoms of various elements that have the same mass number but a different atomic.

Lambang Atom dan Isotop, Isoton, Isobar + konfigurasi PDF
Memahami Definisi Isotop, Isobar dan Isoton dalam Ilmu Kimia Beserta Contoh. 27 Oktober 2023. Yusuf Abdhul Azis. Pada saat pertama kali mengenal istilah isotop, isobar, dan juga isoton ini mungkin kamu akan sedikit bingung bahkan pusing. Apalagi ada sedikit perbedaan antara isotop, isobar dan juga isoton. Meskipun begitu, dewasa ini proses.

Contoh Isotop Isobar Dan Isoton cara mengatasi kaki pegal saat mau tidur
Isotopes and Isobars. Isobar are elements that differ in chemical properties but have the same physical property. So, we can say that isobars are those elements that have a different atomic number but the same mass number. In contrast, Isotopes are those elements having the same atomic number and different mass numbers.