Dalton’s Atomic Theory Know in detail here
At the beginning of the 19th century, the English scientist John Dalton proposed an atomic theory that became the basis for the study of chemistry. His theory contained five main propositions: 1. All matter is comprised of tiny, definite particles called atoms. 2.
What Is The Dalton Atomic Model?
What he learned led him to propose several laws, which are known collectively as Dalton's Atomic Theory or Dalton's Laws: Atoms are small, chemically indestructible particles of matter. Elements consist of atoms. Atoms of an element share common properties. Atoms of different elements have different properties and different atomic weights.

Timeline of the Atom John Dalton 1803, 1805, 1808
The theory of atomism, proposed by Dalton in the early 19th century and derived from meteorological studies, is the foundation for our modern concept of the atom. Plate 5: Elements from John Dalton's A New System of Chemical Philosophy, 1810. Although a schoolteacher, a meteorologist, and an expert on color blindness, John Dalton is best.

Dalton's Atomic Theory ( Read ) Physical Science CK12 Foundation
Model Atom Dalton. John Dalton (1776-1844) adalah ilmuwan yang pertama mengembangkan model atom pada 1803 hingga 1808. Hipotesis Dalton digambarkan dengan model atom sebagai bola pejal seperti tolak peluru. Teori atom Dalton didasarkan pada anggapan: Semua benda terbuat dari atom; Atom-atom tidak dapat dibagi maupun dipecah menjadi bagian lain

Teori Atom Dalton Serta Kelemahan Dan Kelebihannya Rumus Kimia Mobile Legends
John Dalton (born September 5 or 6, 1766, Eaglesfield, Cumberland, England—died July 27, 1844, Manchester) was an English meteorologist and chemist, a pioneer in the development of modern atomic theory.. Early life and education. Dalton was born into a Quaker family of tradesmen; his grandfather Jonathan Dalton was a shoemaker, and his father, Joseph, was a weaver.

PPT TEORI ATOM PowerPoint Presentation, free download ID5126383
Summary. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton based his theory on the law of conservation of mass and the law of constant composition. The first part of his theory states that all matter is made of atoms, which are indivisible.

John Dalton/Democritus Atomic Model
At that size, it takes over 18 million of these atoms, lined up side by side, to equal the width of a human pinky finger (about 1 cm). Figure 2.3.1 2.3. 1: John Dalton was an English scientist who enunciated the modern atomic theory. Dalton studied the weights of various elements and compounds.

Dalton's Atomic Theory YouTube
John Dalton FRS (/ ˈ d ɔː l t ən /; 5 or 6 September 1766 - 27 July 1844) was an English chemist, physicist and meteorologist. He introduced the atomic theory into chemistry. He also researched colour blindness , which he had; as a result, colour blindness is known as Daltonism in several languages.
John Dalton Atomic Theory
Dalton proposed his atomic theory in 1804. The general tenets of this theory are: All matter is composed of extremely small particles called atoms. Atoms cannot be subdivided, created, or destroyed. Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass, and other properties.
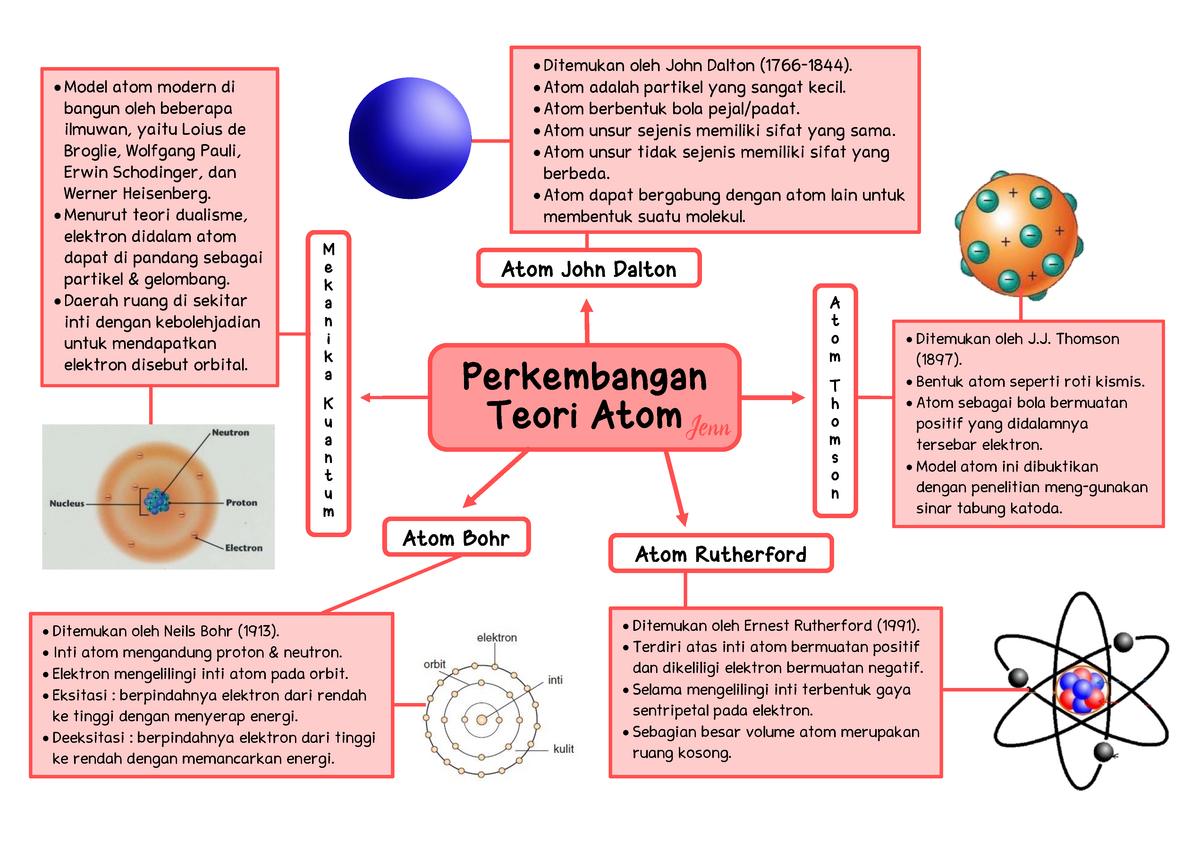
Mindmap Atom Beserta Penjelasan dan Cara Menghapalnya Perkembangan Teori Atom Atom John Dalton
Dalton's atomic model sets up the building blocks for others to improve on. Though some of his conclusions were incorrect, his contributions were vital. He defined an atom as the smallest indivisible particle. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Here's how he defined.

Dalton's Atomic Theory Graphic Education
2. Teori Atom Thomson (Sir Joseph John Thomson) Setelah teori atom Dalton, tokoh perkembangan teori atom selanjutnya adalah teori atom Thomson. Dalam perkembangannya, Thomson memperbaiki kekurangan-kekurangan yang ada pada teori atom Dalton sebleumnya. Pada tahun 1897, Thomson menemukan partikel yang bermuatan negatif dan disebut dengan elektron.

5 Teori Struktur Atom dan Penjelasannya dari Masa ke Masa
Tercatat teori atom milik John Dalton adalah teori tertua yang membahas atom. Sejarah Teori Atom John Dalton. Awal mulanya John Dalton menyebutkan bahwa atom dapat diibaratkan sebagai blok bangunan yang tersusun untuk membentuk struktur kimia. Setelahnya John Dalton mengembangkan sebuah hukum yang dikenal dengan hukum perkembangan ganda yang.

Biografi John Dalton, Kisah 'Bapak Kimia' Penemu Teori Atom Modern
Dalton's atomic theory was a scientific theory on the nature of matter put forward by the English physicist and chemist John Dalton in the year 1808. It stated that all matter was made up of small, indivisible particles known as 'atoms'. All substances, according to Dalton's atomic theory, are made up of atoms, which are indivisible and.
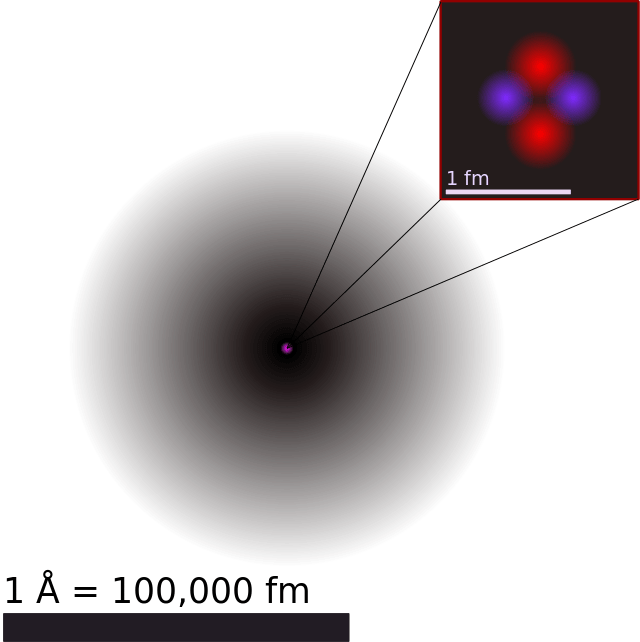
What Is John Dalton's Atomic Model? Universe Today
Teori atom Dalton dikembangkan oleh John Dalton pada tahun 1803 hingga 1809. Lewat teori ini, Dalton menyatakan atom adalah zat yang tidak dapat dibagi lagi menjadi zat-zat yang lebih sederhana atau lebih kecil. Teori atom Dalton adalah pengembangan model atom pertama di dunia. Awalnya, teori atom diperkenalkan pertama kali oleh filsuf Yunani.
---teachoo-01.jpg)
Dalton's Atomic Theory Postulate, Limitations [Teachoo]
Many consider 2008 the 200th anniversary of atomic theory, John Dalton's momentous theory of the nature of matter. Dalton (1766-1844) proposed that all matter in the universe is made of indestructible, unchangeable atoms—each type characterized by a constant mass—that undergo chemical reactions by joining with and separating from each other.

Atomic theories
John Dalton and the development of the atomic theory. By far Dalton's most influential work in chemistry was his atomic theory. Attempts to trace precisely how Dalton developed this theory have proved futile; even Dalton's own recollections on the subject are incomplete. He based his theory of partial pressures on the idea that only like.