Difference Between Polar and Nonpolar Molecules Definition, Formation, Properties, Examples
Molekul polar dapat berinteraksi secara hidrofilik (menarik air) atau hidrofobik (menolak air) tergantung pada sifat dan susunan atom penyusunnya. Molekul polar dapat mengalami interaksi kuat antara satu sama lain, seperti gaya tarik Van der Waals, gaya dipol-dipol, dan ikatan hidrogen. Contoh molekul polar termasuk air (H₂O), amonia (NH₃.

Moleküllerin Polarlık ya da Apolarlığı konu anlatımı ders notu 9.sınıf kimya
Updated on January 20, 2020 A polar molecule is a molecule containing polar bonds where the sum of all the bond's dipole moments is not zero. Polar bonds form when there is a difference between the electronegativity values of the atoms participating in a bond.
MOLEKUL POLAR & NON POLAR YouTube
A polar molecule is a chemical species in which the distribution of electrons between the covalently bonded atoms is not even. Polarity is a description of how different the electrical poles of a molecule are.

Bentuk molekul berikut yang bersifat polar adalah... (bis...
3. Add all of the bonds. Use the octet rule to determine the number and type of bonds present. Each atom'svalence shell should contain 8 electrons for the molecule to be stable. Some atoms may be double or triple bonded to achieve this. [3] In a water molecule, add a single bond from the oxygen to both hydrogens.
VSEPR Theory and Polarity Kyle's Digital Portfolio
Atoms consist of a single nucleus with a positive charge surrounded by a cloud of negatively charged electrons.When atoms approach one another closely, the electron clouds interact with each other and with the nuclei. If this interaction is such that the total energy of the system is lowered, then the atoms bond together to form a molecule. Thus, from a structural point of view, a molecule.

Molekul Polar dan Molekul Non Polar YouTube
A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. A diatomic molecule that consists of a polar covalent bond, such as HF, is a polar molecule.
PPT ELECTRONEGATIVITY POLAR BONDS MOLECULAR POLARITY PowerPoint Presentation ID4261659
Polar materials tend to be more attracted to and are more soluble in polar solvents. Nonpolar materials tends to be attracted to and are more soluble in nonpolar materials. Polar molecules are those that possess regions of positive and negative charge. Water is an example of a polar material. The type of bonds it has, when coupled with its.
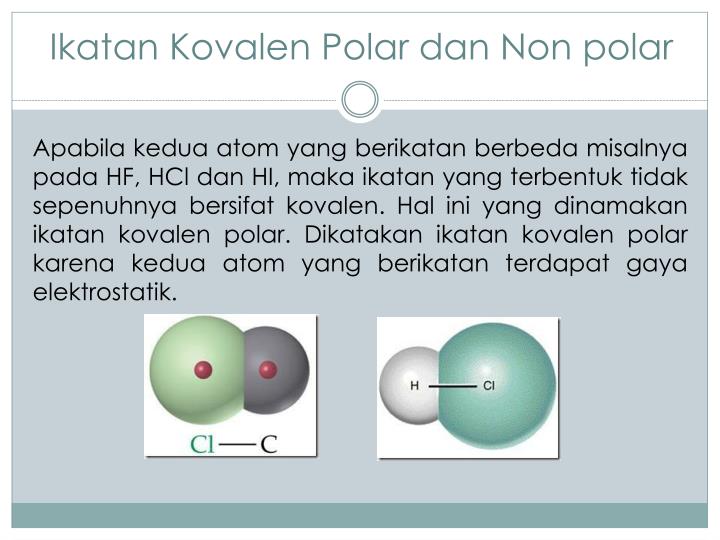
Ikatan Kovalen Polar Dan Nonpolar Beserta Contoh Ikatannya Rumus Kimia Sexiz Pix
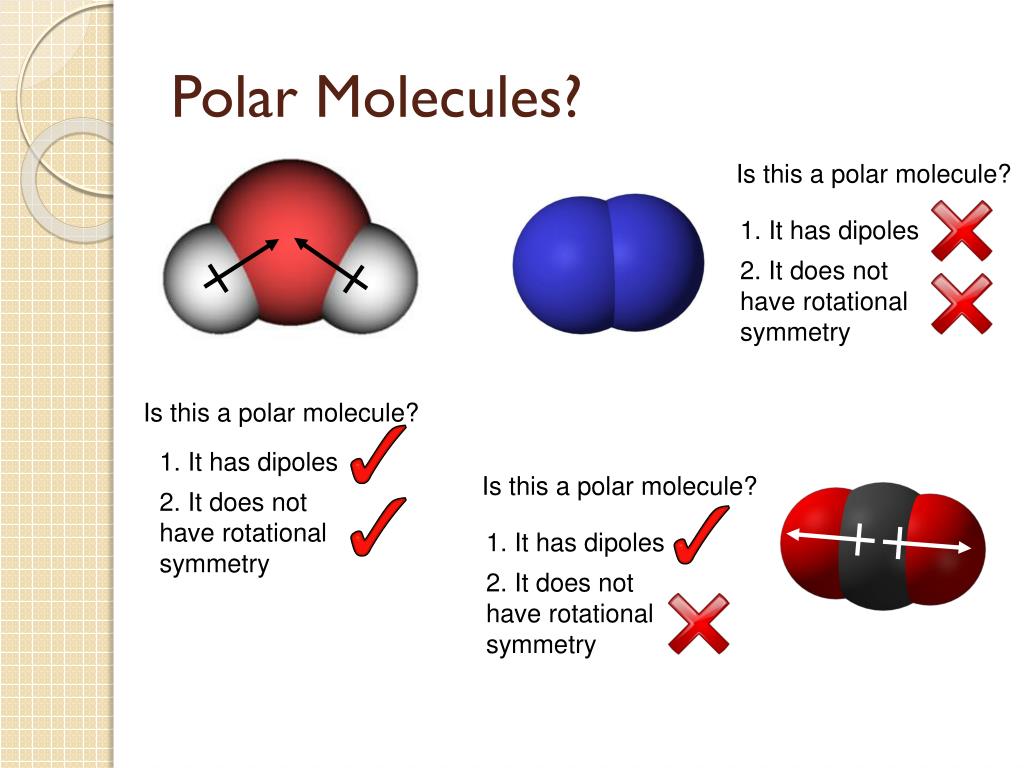
Polar Molecules. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. A diatomic molecule that consists of a polar covalent bond, such as \(\ce{HF}\), is a polar molecule. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole.
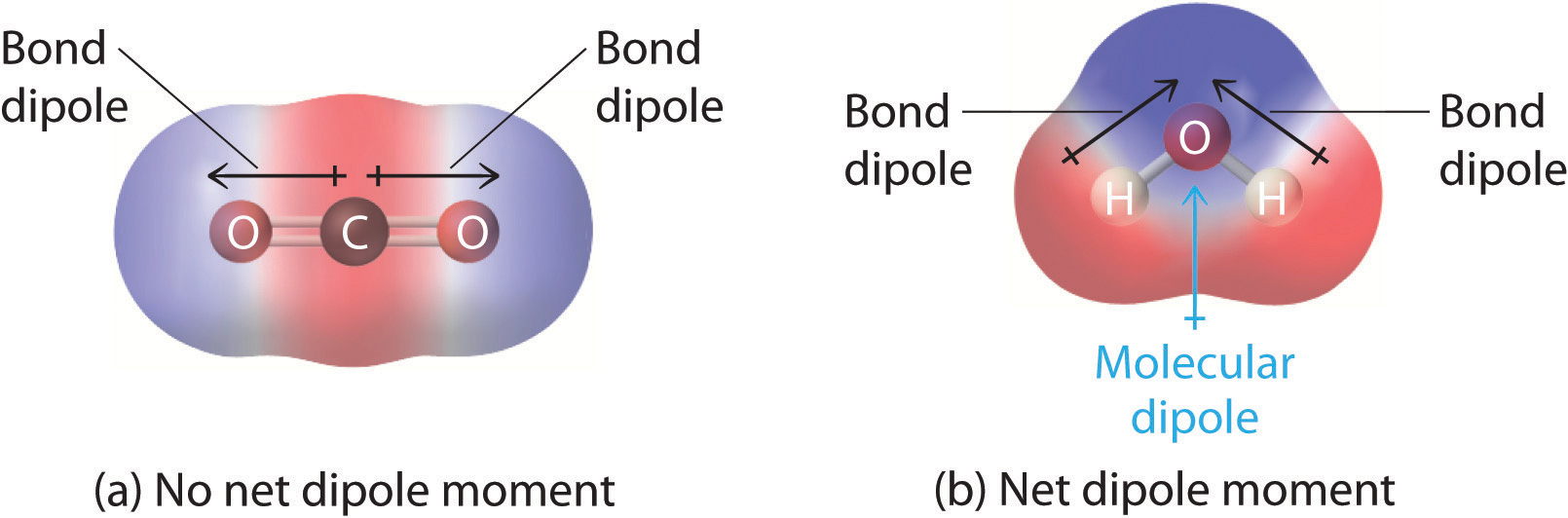
2.2 Polar Covalent Bonds Dipole Moments Chemistry LibreTexts
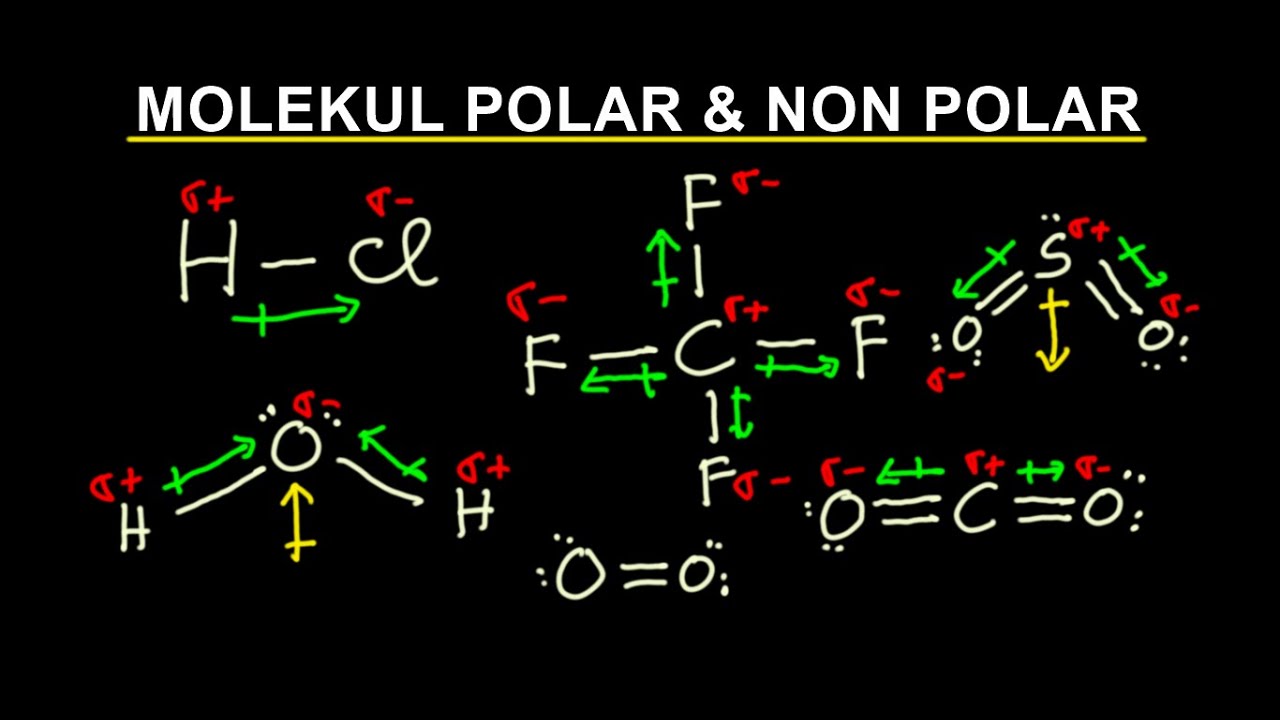
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. The electrons are opposite Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms.

Contoh Senyawa Kovalen Polar
Non-polar molecules: Hydrocarbons (gasoline, toluene), homo-nuclear diatomic molecules (O 2, N 2, Cl 2, H 2, etc.), noble gases, benzene, methane, ethylene, carbon tetrachloride How to Determine if a Molecule is Polar Or Nonpolar. Start by drawing its Lewis structure. This rule applies to all molecules except hydrocarbons and molecules with two atoms of the same element.

Bentuk molekul 10 SMA (Menentukan kepolaran senyawa dari bentuk molekul) YouTube
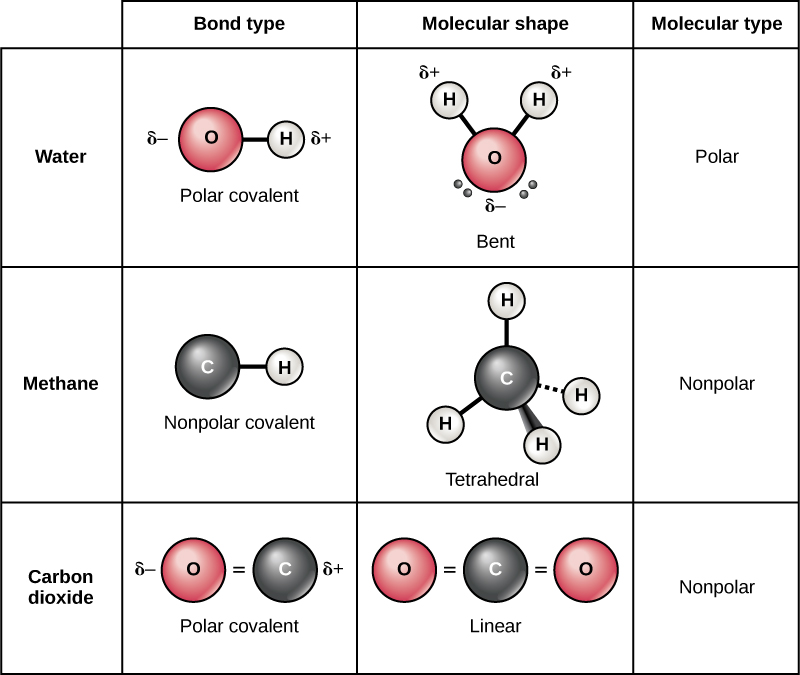
Molekul polar adalah molekul dengan ikatan polar yang dipolnya tidak dibatalkan. Artinya, mereka adalah molekul yang menghadirkan momen dipol permanen, menghasilkan perbedaan muatan listrik pada ikatan molekul. Contoh paling umum dari molekul polar adalah air (H 2 O).
:max_bytes(150000):strip_icc()/H20-58e1ef103df78c5162595632.jpg)
Why Is Water a Polar Molecule?
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
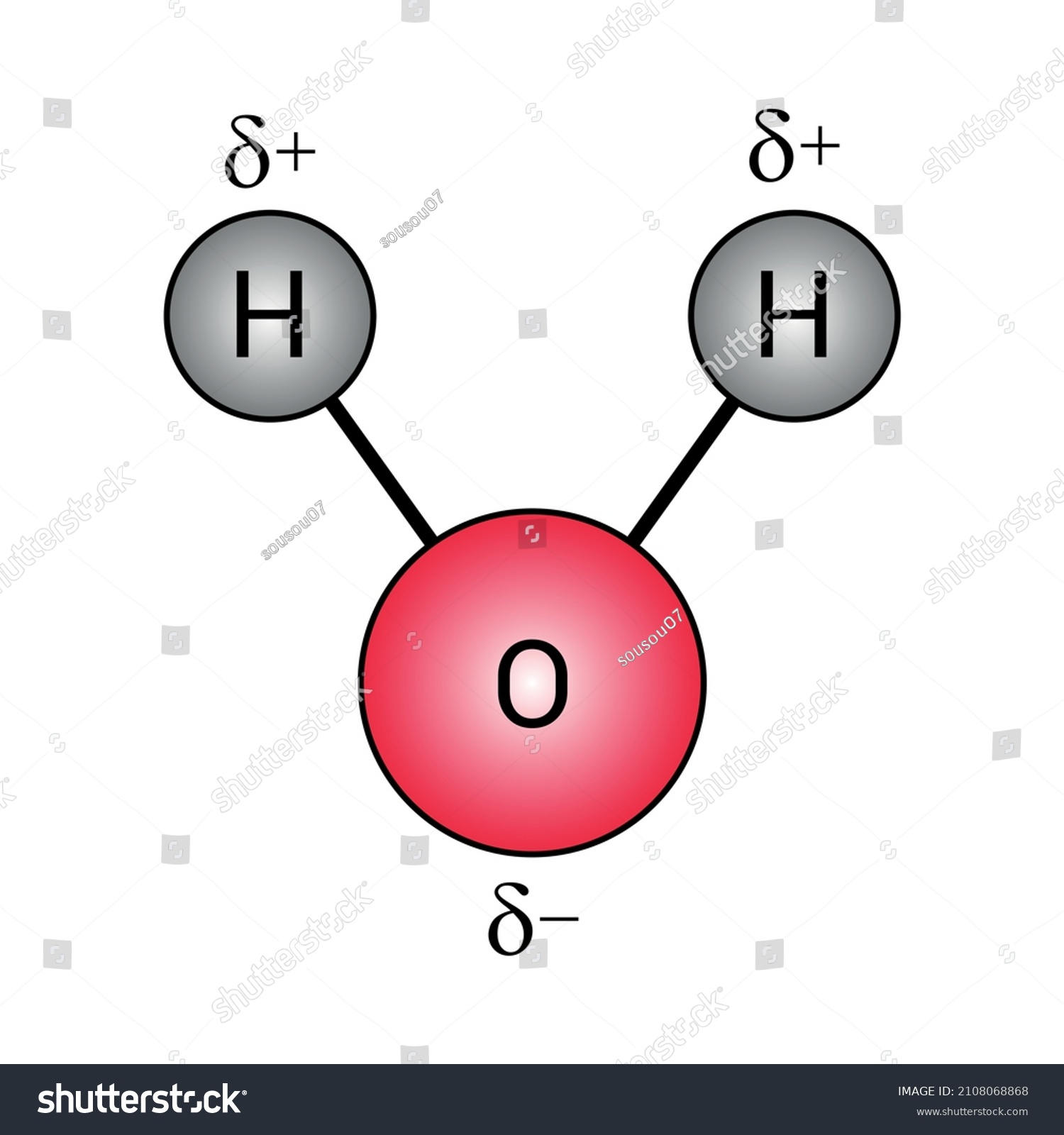
Polar Covalent Bonds Water Molecules H2o Stock Vector (Royalty Free) 2108068868
About Transcript Like bonds, molecules can also be polar. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge.

Polar and Nonpolar Covalent Bonds Characteristics & Differences Still Education
Atom oksigen dalam molekul air memiliki elektronegatifitas yang lebih besar dari pada atom hydrogen yang terikat secara kovalen, menghasilkan pergeseran dipol di mana ikatan tersebut berbobot positif pada ujung hydrogen. Baca juga: Menentukan Kadar Masing-Masing Unsur dalam Senyawa Aspirin C9H8O4

Polare und unpolare Moleküle Free Press
Ikatan Kimia (8) | Cara Menentukan Molekul Polar dan Non Polar | Kimia Kelas 10 Kimatika 103K subscribers Subscribe Subscribed 2.3K 98K views 3 years ago Kimia kelas 10 | Ikatan Kimia.

Hem apolar hem de polar kovalent bağ nasıl anlaşılır örnekle açıklar mısınız
Answer. 4.10: Polar Molecules is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by LibreTexts. The molecular polarity of a diatomic molecule is determined by the bond polarity. The polarity of molecules with more than one bond must be determined by first identifying the molecular structure and..