IUD SMB TCu380Ag ("Nova T" type) with copper and silver / ORDER WITH BIG DISCOUNTlook here / T
NOVA-T is indicated for intrauterine contraception in gynecologically normal women of childbearing age. The pregnancy rate with NOVA-T is 1.26 per 100 woman-years. After removal of NOVA-T, fertility is promptly restored. Duration of use: NOVA-T can be left inserted for a maximum of 30 months. If continued contraception is desired by
-0297.jpg)
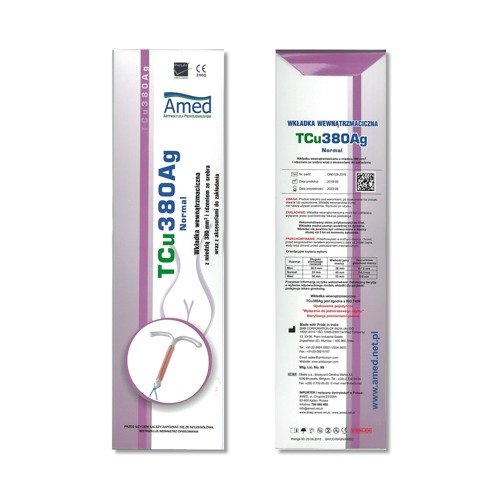
SMB TCu 380Ag Intrauterine 380Ag Devices, Nova T, Silver IUD
Background. We describe the rationale and protocol for a randomized noninferiority controlled trial (RCT) to determine if the Flexi-T380(+) copper intrauterine contraceptive device (IUD) is comparable in terms of effectiveness and expulsion rates to the most common Canadian IUD currently in use, NovaT-200, when placed immediately after a first-trimester abortion.

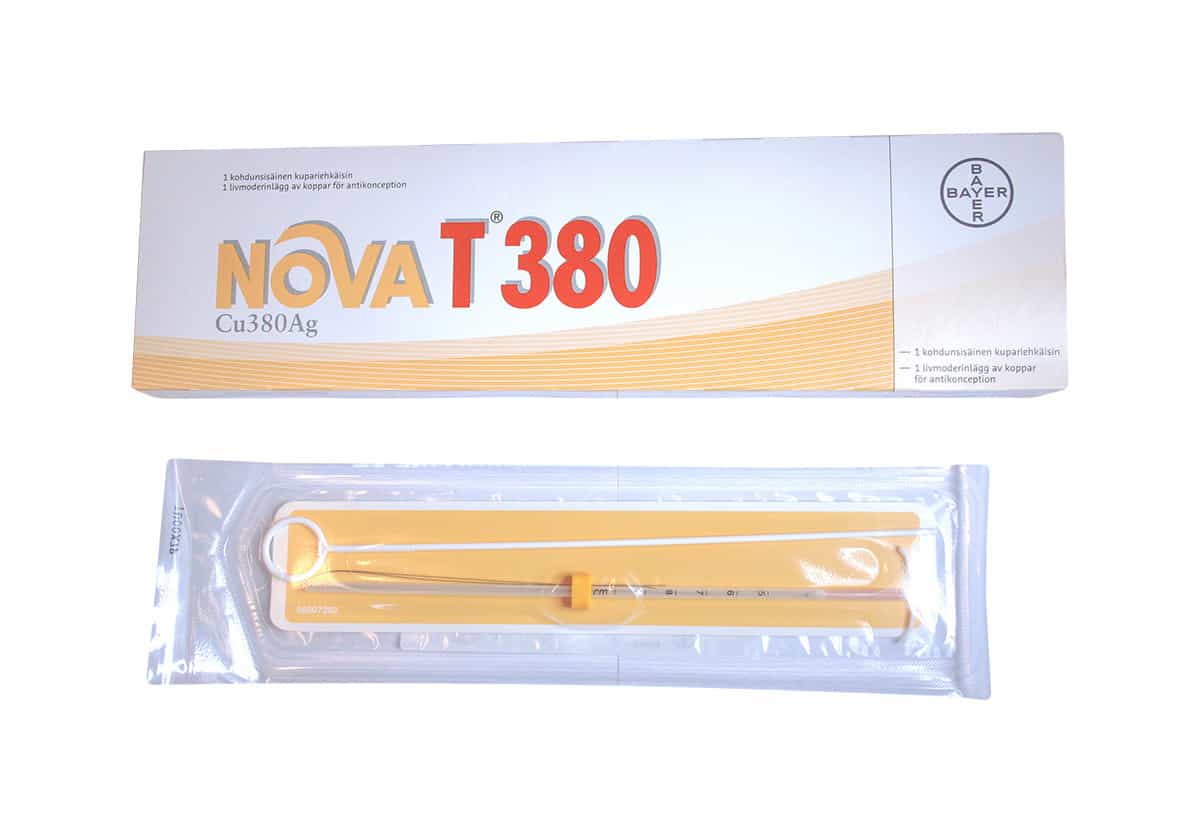
IUCD NovaT 380 IUD
The Nova-T IUD is being widely used in Western Europe, Canada, and some parts of Asia; numerous studies based on comparative trials with other types of IUDs have been reported. The results were consistent among most studies, but conflicting among others. This review paper revealed that, in general, the Nova-T IUD is a safe, effective, and.
NOVAPLUS IUD Range
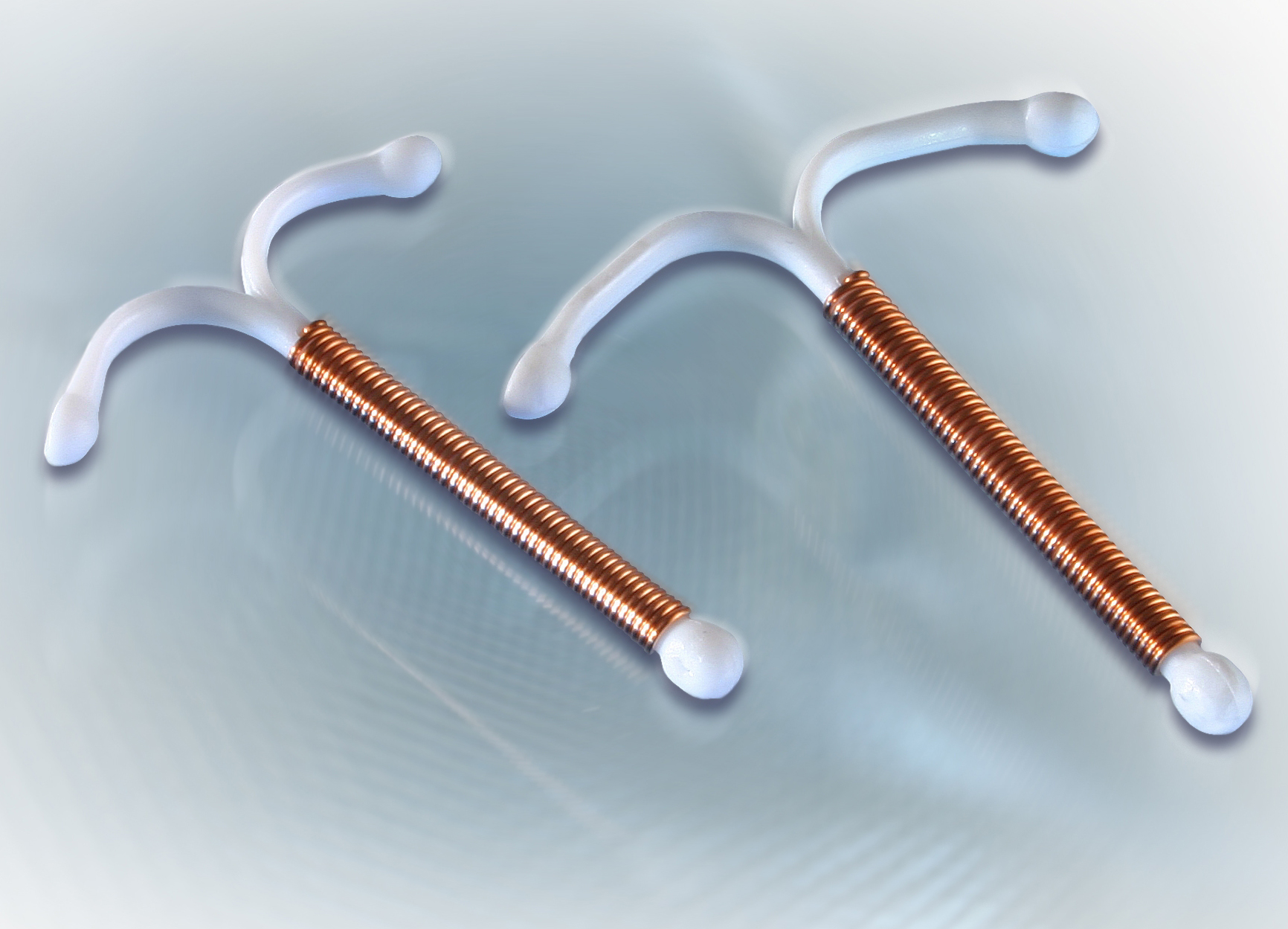
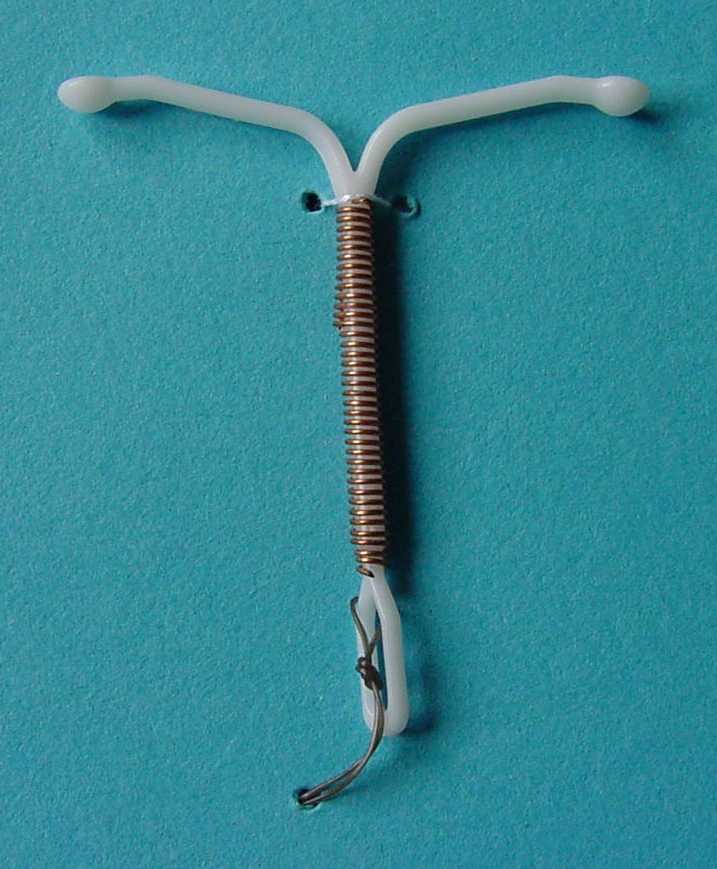
Section snippets Device. NOVA T®380 is identical with NOVA T®200 (a modified T-shape plastic frame with flexible arms and silver-cored copper wire wound around the vertical stem), except for the increased copper surface (380 mm 2), the diameter of the copper thread (0.4 mm with a 0.1 mm silver core) and the length of the loop (Fig. 1).The total length of the horizontal arms is about 32 mm.
D.I.U Nova T 380 De Cobre
Another trial assigned women to the levonorgestrel IUD or Nova T; more women with the Nova T stopped use due to pregnancy. A subanalysis showed more IUDs came out when inserted right after abortion or miscarriage rather than later. Seven trials looked at inserting the IUD right away. From two large trials, the TCu 220C was better than the.

Nova T380 Cu380Ag IUD (IntraUterine Device) (Single) Hillcroft Supplies
The Nova-T 200 IUD is safe and cost-effective and should continue to be offered as a contraceptive option for properly selected women. Although poorly tolerated by nulliparous women, it proved to be very satisfactory for multiparous women, particularly nursing mothers and those over 40 years of age.
IUD SMB TCu380Ag ("Nova T" type) with copper and silver / ORDER WITH BIG DISCOUNTlook here / T
T: 1.800.667.3425 (SK) | 966.6340 (Saskatoon)| [email protected] www.medSask.usask.ca Comparison of Copper Intrauterine Devices Available in Canada Brand Name. TT similar to that of Nova-T UT similar to that of MirenaNulliparous 29.9 34 5 Nulliparous or < 7 cm uterus 64

IUCD NovaT 380 IUD
In an open, single-group, phase III clinical trial of 5 years, the clinical performance of NOVA T 380 was investigated in three centers. The device having a higher copper surface of 380 mm (2) is a modification of NOVA T. A total of 400 voluntary women were enrolled in the study. The mean age was 31.4 years (SD 5.5) with the minimum of 18 and.

NovaT Copper IUD Birth Control 13 Oct 2015 Mommy and Baby Approved YouTube
A copper intrauterine device (IUD), also known as an intrauterine coil or copper coil or non-hormonal IUD, is a type of intrauterine device which contains copper. It is used for birth control and emergency contraception within five days of unprotected sex. It is one of the most effective forms of birth control with a one-year failure rate around 0.7%. The device is placed in the uterus and.
IUD NOVA T 380 Nova T Cu 380 Ag IntraUterine Device 1'S Lazada Indonesia
While IUD size is one factor, our findings may be due to other differences between the products. For example, the shorter arms of the NT380-Mini splay outward from the stem and flex upward (the Nova-T design used on hormonal IUDs), whereas the TCu380A arms are rigidly perpendicular to the stem . Also, the TCu380A has copper on the arms/stem.
intrauterin
The Nova-T IUD showed favorable results in some studies, but showed deteriorated efficacy after three years of use in others; more studies are needed. Studies show that the IUD that daily releases 20 μg levonorgestrel (LNG-20) is associated with the highest efficacy in preventing accidental pregnancy among the four devices, but it has a.

EUROGINE Sterile Intrauterine Spiral IUD to Anchor and Y in Copper Silver Gold Novaplus T 380 Ag
In an open, single-group, phase III clinical trial of 5 years, the clinical performance of NOVA T®380 was investigated in three centers. The device having a higher copper surface of 380 mm2 is a modification of NOVA T®. A total of 400 voluntary women were enrolled in the study. The mean age was 31.4 years (SD 5.5) with the minimum of 18 and the maximum of 44 years.

Museum für Verhütung und Schwangerschaftsabbruch
NOVA T 380 . Intrauterine device (IUD) COMPOSITION: NOVA T 380 is an intrauterine device made of polyethylene and wound with copper wire with a silver core. The surface area of the copper is 380 mm. 2. The polyethylene body, shaped as a modified T is impregnated with barium sulphate. Removal threads, pigmented with iron oxide are attached to.

STÉRILET NOVAT Polymed Chirurgical Inc.
One patient reported an adverse event. During the check-up, the gynecologist detected a dislocation and partial perforation of the IUD (NOVA T 380) into the fundal myometrium without any signs of PID. The patient was asymptomatic; no surgical manipulation was reported. The IUD was removed and a new device (NOVA T 380) was implanted.
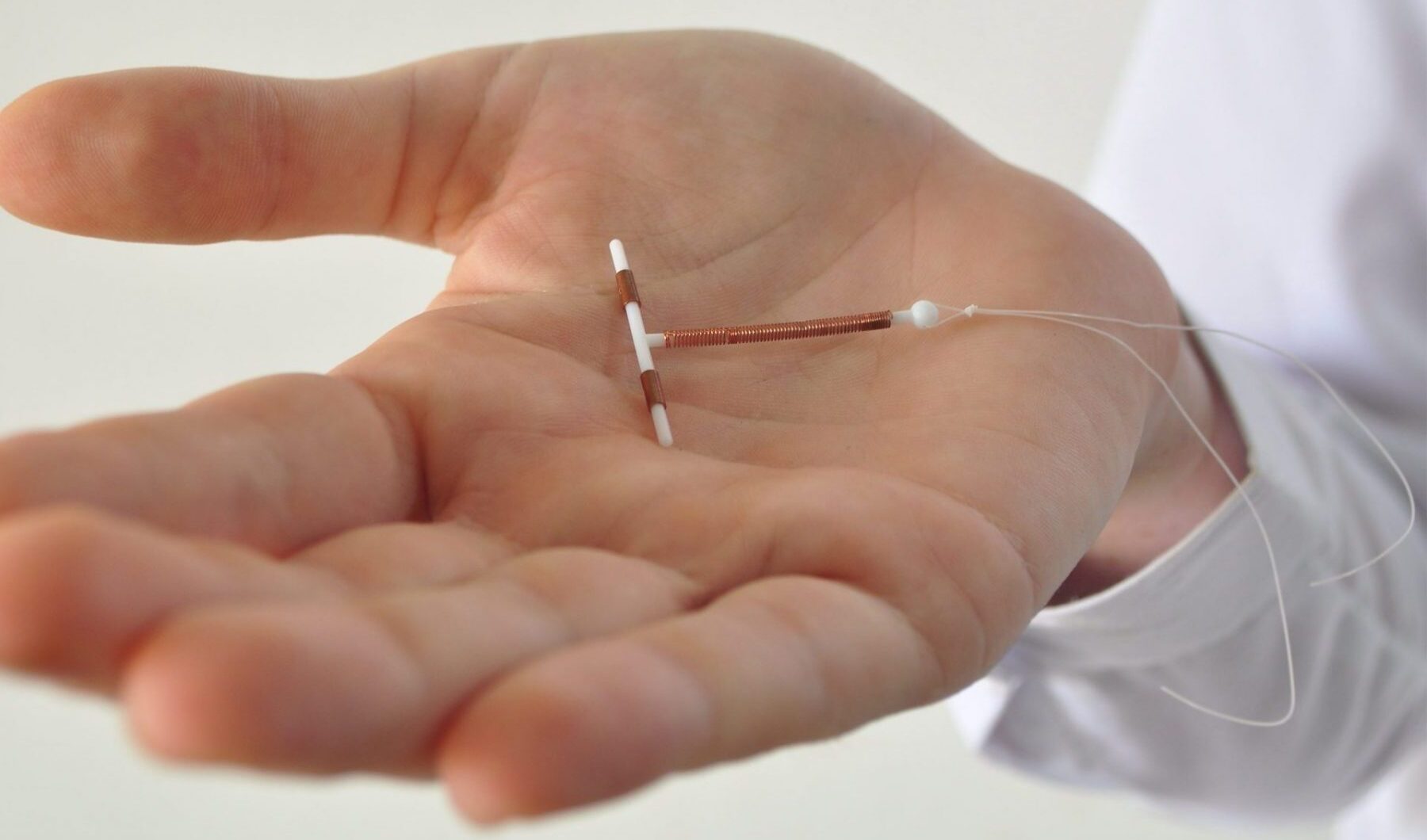
Nova T If you do not want hormones at all IUD התקנים תוך רחמיים
The Nova-T IUD is being widely used in Western Europe, Canada, and some parts of Asia; numerous studies based on comparative trials with other types of IUDs have been reported. The results were consistent among most studies, but conflicting among others.This review paper revealed that, in general, the Nova-T IUD is a safe, effective, and acceptable device for contraception.

Nova T 380 Diu a farmácia online
Before inserting an intra-uterine contraceptive device, inform patients that perforation occurs in approximately 1 in every 1000 insertions and signs and symptoms include: severe pelvic pain after insertion (worse than period cramps); pain or increased bleeding after insertion which continues for more than a few weeks; sudden changes in periods;