
Rumus Delta S Kimia Bit CDN
Dr. Hodge Sanitarium. Dr. Hodge Sanitarium, 424 Pine Ave., Niagara Falls, was operated by doctors William and John Hodge, who were brothers. They were Niagara County residents who grew up on a.

What Is Delta Tf in Chemistry
100, 0128 ∘ C. 5. 100, 0234 ∘ C. Belajar Modul, Rumus, & Soal Sifat Koligatif Larutan dan kuis interaktif. Dapatkan Modul, Rumus, & Soal Sifat Koligatif Larutan lengkap di Wardaya College.

Rumus tb mektek 1
بِسْــــــــــــــــــمِ اللهِ الرَّحْمَنِ الرَّحِيْمِ. Assalamualaikum w.r.b. teman-teman. Slamat datang dibisakimia.com insyaallah pasti bisa. Berikut ialah Rumus Serta Soal dan Pembahasan SIFAT KOLIGATIF LARUTAN. Jadi ini adalah bab I mapel kimia dikelas XII .

rumus z score bb/tb Informasi Seputar Gizi
Overview. Tuberculosis (TB) is a serious illness that mainly affects the lungs. The germs that cause tuberculosis are a type of bacteria. Tuberculosis can spread when a person with the illness coughs, sneezes or sings. This can put tiny droplets with the germs into the air. Another person can then breathe in the droplets, and the germs enter.

Rumus Kenaikan Titik Didih Hot Sex Picture
3.5: Loss Tangent. Page ID. Steven W. Ellingson. Virginia Polytechnic Institute and State University via Virginia Tech Libraries' Open Education Initiative. In Section 3.3, we found that the effect of loss due to non-zero conductivity σ σ could be concisely quantified using the ratio. ϵ′′ ϵ′ = σ ωϵ (3.5.1) (3.5.1) ϵ ″ ϵ.
What is the need and applicability of Van't Hoff Factor? Explain the conditions where delta Tb
ΔTb = m × Kb. Titik didih larutan = 100 °C + 5,2 °C = 105,2 °C. Titik beku larutan = 0 °C - 18,6 °C = -18,6 °C. 2. Titik beku larutan 64 gram naftalena dalam 100 gram benzena adalah 2,91 °C. Jika titik beku benzena 5,46°C dan tetapan titik beku molal benzena 5,1 °C, maka. tentukan massa molekul relatif naftalena! 3.

Termokimia Pengertian Persaman Reaksi Rumus Dan Contoh Soal Soal Sexiz Pix
From thermodynamics, the heat capacity is defined as Cp = (dH dT)P C p = ( d H d T) P. That means we can calculate the heat required to change the temperature of some material from the following integral: H2 −H1 = Q = ∫T2 T1 Cp(T)dT H 2 − H 1 = Q = ∫ T 1 T 2 C p ( T) d T. In the range of 298-1200K, the heat capacity of CO 2 is given by.

√ Rumus Delta T
Berikut penjelasan kelompok sifat koligatif: 1. Koligatif Larutan - Penurunan tekanan uap jenuh (Δ Tp ) Penurunan tekanan uap jenuh adalah selisih tekanan uap pelarut murni dan tekanan uap larutan . tabel penurunan tekanan uap jenuh larutan non elektrolit dan elektrolit ↓. Uraian. Larutah non elektrolit.
Model performance and delta with the 3layer DNN) Download Scientific Diagram
Specific Heat Formula. When heat energy is added to a substance, the temperature will change by a certain amount. The relationship between heat energy and temperature is different for every material, and the specific heat is a value that describes how they relate. heat energy = (mass of substance) (specific heat) (change in temperature) Q = mc∆T.
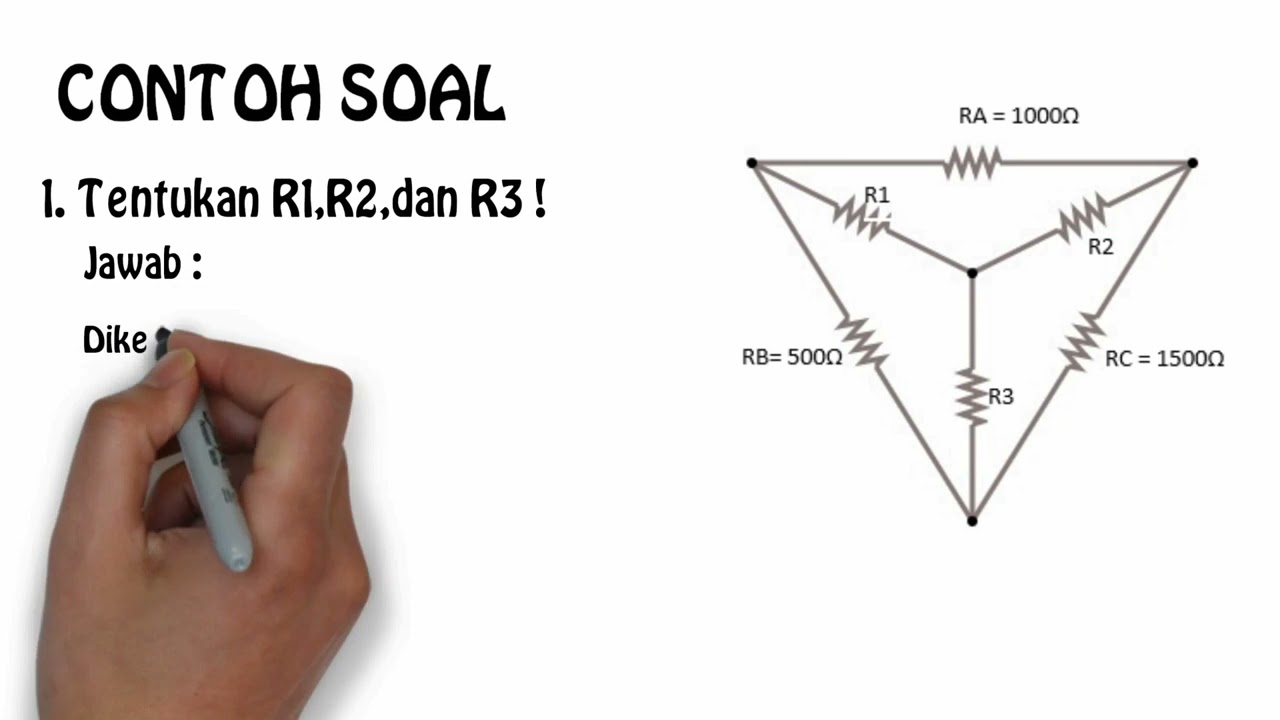
cara menghitung rumus star delta kelistrikan YouTube
So, from the first and 2nd laws of thermodynamics applied to this control volume system, $$\dot{q}-\dot{w}=\Delta h$$ and $$\frac{\dot{q}}{T}+\dot{\sigma}=\Delta s$$ where $\dot{q}$ is the heat flow per mole from the reservoir to the control volume, $\dot{w}$ is the shaft work per mole done on the surroundings, and $\dot{\sigma}$ is the entropy.
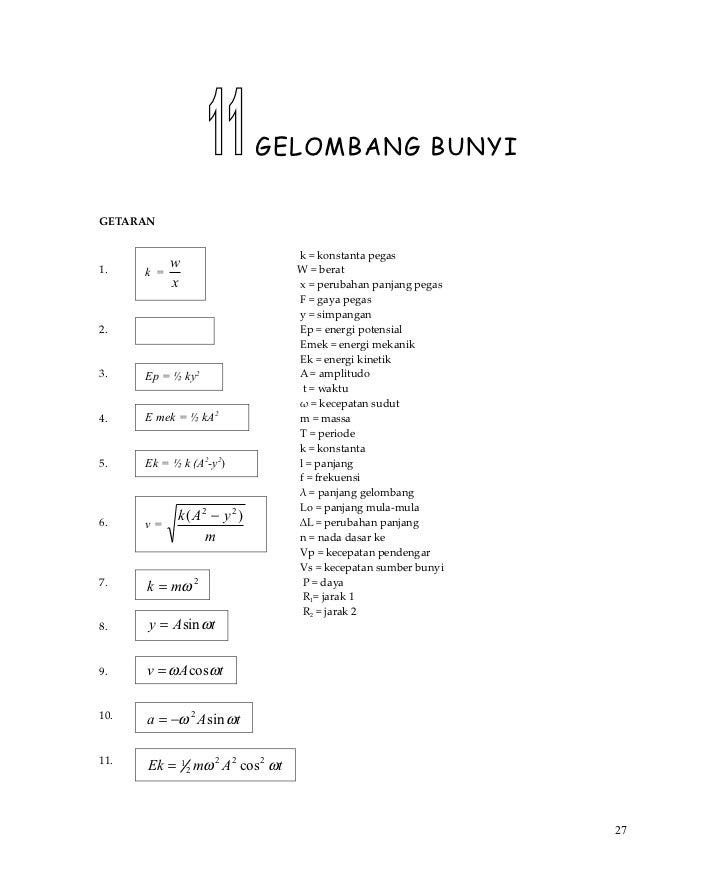
√ Rumus Delta T
Boiling Point Elevation. The boiling points of solutions are all higher than that of the pure solvent. Difference between the boiling points of the pure solvent and the solution is proportional to the concentration of the solute particles: ΔTb = Tb(solution) −Tb(solvent) = Kb × m (1) (1) Δ T b = T b ( s o l u t i o n) − T b ( s o l v e n.
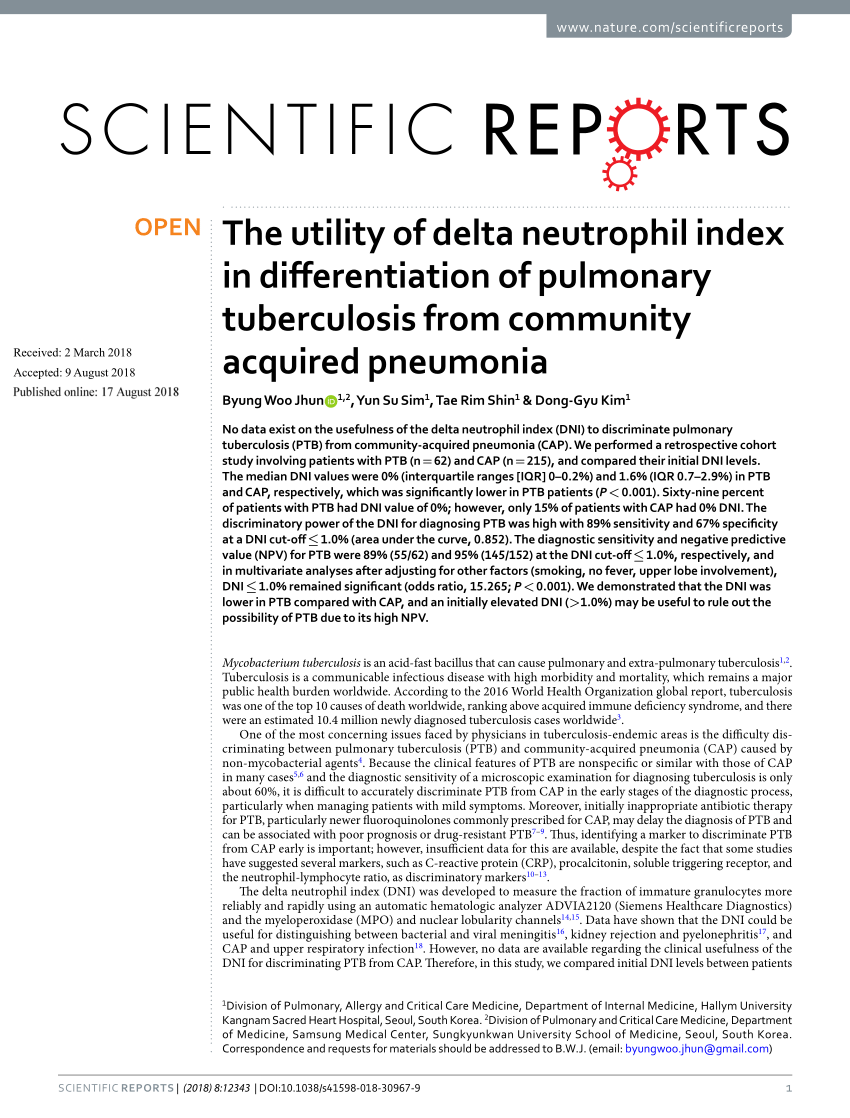
(PDF) The utility of delta neutrophil index in differentiation of pulmonary tuberculosis from
Hitunglah Disini yang pertama kenaikan titik didih larutan Kemudian yang kedua titik didih larutan tersebut dengan mr-nya 342 KB air = 0,512 derajat Celcius kg per mol atau = 0,52 derajat Celcius per molal air = 100 derajat Celcius Nah di sini sukrosa merupakan larutan nonelektrolit di mana nilainya atau faktor van't hoff nya = 1 Delta TB atau.
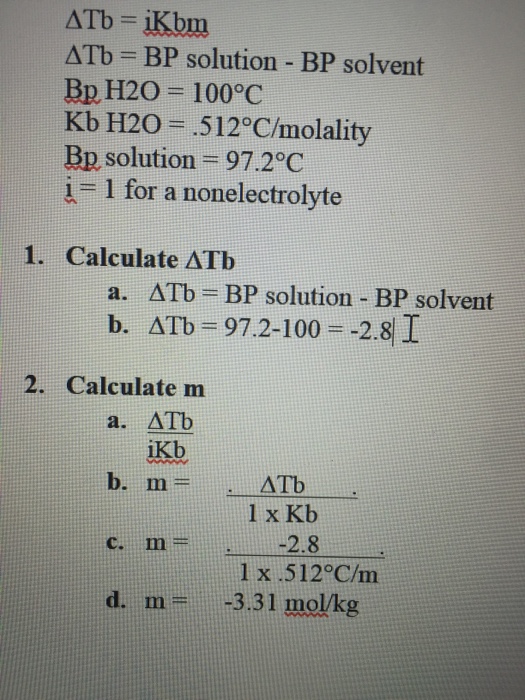
Solved Delta Tb = iKbm Delta Tb = BP solution BP solvent
Rumus delta TB adalah rumus yang digunakan untuk menghitung perbedaan suhu badan atau temperatur tubuh. Rumus ini sangat penting untuk mengetahui seberapa besar perbedaan suhu badan seseorang yang sedang sakit atau dalam kondisi tertentu. Suhu badan normal manusia adalah sekitar 36,5-37,5 derajat Celsius. Namun, jika seseorang mengalami demam.
What is the need and applicability of Van't Hoff Factor? Explain the conditions where delta Tb
Absolute Delta. If you have a random pair of numbers and you want to know the delta - or difference - between them, just subtract the smaller one from the larger one. For example, the delta between 3 and 6 is (6 - 3) = 3. If one of the numbers is negative, add the two numbers together. The operation looks like this: (6 - {-3}) = (6 + 3) = 9.

√ Rumus Delta T
ΔTb = Tb larutan −Tb pelarut murni. Rumus di atas berlaku untuk larutan nonelektrolit. b. ΔTb tidak berlaku untuk larutan yang mudah menguap.. Misalkan = delta yaa Tb = m . Kb Tb/ m = kb Dengan: Tb = kenaikan titik didih m = molalitas kb = tetapan kenaikan titik didih #cmiiw 27 November 2020 pukul 00.32

Rumus Zscore TB/U & PB/U Informasi Seputar Gizi
Meskipun sifat koligatif melibatkan larutan, sifat koligatif tidak bergantung pada interaksi antara molekul pelarut dan zat terlarut, tetapi bergatung pada jumlah zat terlarut yang larut pada suatu larutan. Sifat koligatif terdiri dari penurunan tekanan uap, kenaikan titik didih, penurunan titik beku, dan tekanan osmotik. 1. Penurunan Tekanan Uap.