
Menentukan nilai kelarutan dari Ksp Kelarutan 2 YouTube
The equilibrium constant for a dissolution reaction, called the solubility product ( Ksp ), is a measure of the solubility of a compound. Whereas solubility is usually expressed in terms of mass of solute per 100 mL of solvent, Ksp is defined in terms of the molar concentrations of the component ions. In contrast, the ion product ( Q) describes.

Kelarutan (s) dan Hasil Kali Kelarutan (Ksp) Soal dan Pembahasan KIMIA KELAS 11 YouTube
The dissociation equation for PbF 2 and the corresponding K s p expression. PbF 2 ( s) ⇄ Pb 2 + ( a q) + 2 F − ( a q) K s p = [ Pb 2 +] [ F −] 2. The steps above will be followed to calculate the K s p for PbF 2. Step 2: Solve. molar solubility 0.533 g L × 1 mol 245.20 g = 2.17 × 10 − 3 M. The dissociation equation shows that for.
2.jpg)
Cara Menghitung Ksp Sinau
Practice Questions (please show all work) 1. Given the Ksp for Fe F2 is 2.36 x 10^(-6), find the solubility of the Fe and F2 ions in mols/L or molarity (M).

Kelarutan dan ksp soal kimia SMA YouTube
Contoh Soal Kelarutan dan Hasil Kali Kelarutan (KSP) dan Pembahasan Contoh Soal 1: Hitunglah kelarutan Cu(OH) 2 dalam satuan g/L, jika diketahui K sp Cu(OH) 2 = 2,2 × 10 −20. Pembahasan: Contoh Soal 2: Hitunglah kelarutan molar PbI 2 dalam larutan KI 0,1 M. (K sp PbI 2 = 7,1 × 10 −9) Pembahasan: Dalam larutan, KI akan terdisosiasi menjadi.
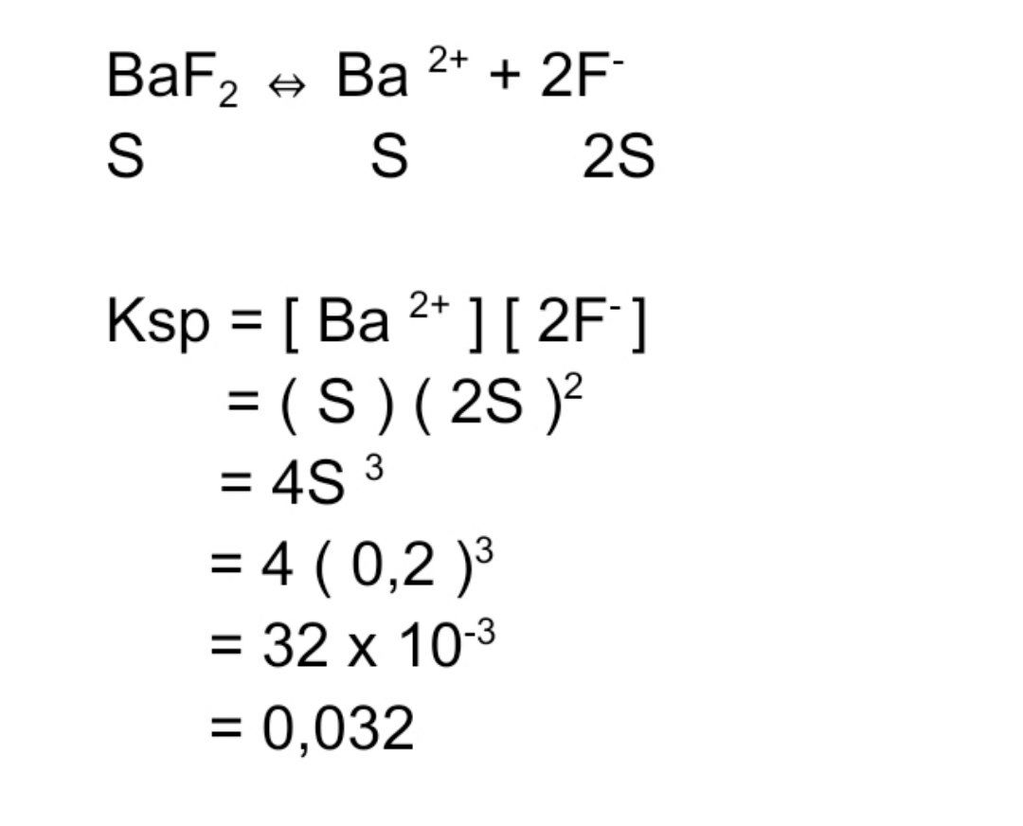
Tentukan besarnya Ksp senyawa berikut. 350 gram Ba...
K_(sp) is called solubility product constant, or simply solubility product. In general, the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. Here's an example to better demonstrate the concept. Let's consider the saturated solution of silver chloride (AgCl.
Tuliskanlah rumus kelarutan(Ksp) dari senyawa beri...
Pengertian KSP Kimia.. Kemudian, dilakukan perhitungan dengan menggunakan rumus yang sesuai untuk senyawa tersebut. Hasil perhitungan adalah nilai KSP yang menunjukkan tingkat kelarutan senyawa tersebut dalam air. Contoh Soal Penghitungan KSP. Misalnya, diberikan data kelarutan AgCl sebesar 1,6 x 10^-5 M. Berapa nilai KSP dari AgCl?

Soal Tentukan rumus Ksp dari CuCl!
Table of Contents. The equilibrium constant for a solid substance dissolving in an aqueous solution is the Ksp. The level at which a solute can be dissolved in the solution is represented by Ksp. The higher the Ksp value of a substance, the more it is soluble. Consider the general dissolution reaction in the solution.

Hasil Kali Kelarutan Ksp
Hai sobat trivia,Di video kali ini berisi penjelasan Bab Hasil Kali Kelarutan mengenai konsep kelarutan dan rumus Ksp. Penjelasan materi di video ini juga di.

Cara Mentukan Rumus Ksp Kimia Bit CDN
The solubility product constant is the equilibrium constant for the dissolution of a solid substance into an aqueous solution. It is denoted by the symbol Ksp. The solubility product is a kind of equilibrium constant and its value depends on temperature. Ksp usually increases with an increase in temperature due to increased solubility.

How To Calculate The Ksp
Here's an example: The K s p value of A g 2 S O 4 ,silver sulfate, is 1.4× 10 - 5. Determine the molar solubility. First, we need to write out the dissociation equation: K s p = [ A g +] 2 [ S O 4 2] Next, we plug in the K s p value to create an algebraic expression. 1.4× 10 - 5 = ( 2 x) 2 ( x) 1.4× 10 - 5 = 4 x 3.

Image result for ksp chemistry equation Science chemistry, High school chemistry, Chemistry
First, write out the Ksp expression, then substitute in concentrations and solve for Ksp: CaF 2 ( s) ↽ − − ⇀ Ca 2 + ( aq) + 2 F − ( aq) A saturated solution is a solution at equilibrium with the solid. Thus: K sp = [ Ca 2 +] [ F −] 2 = ( 2.1 × 10 − 4) ( 4.2 × 10 − 4) 2 = 3.7 × 10 − 11. As with other equilibrium constants.

Soal Tentukan rumus Ksp dari CuCl!
Tetapan Hasil Kali Kelarutan (Ksp) - RumusKimia.net kali ini akan berbagi materi kimia tentang Rumus Tetapan Hasil Kali Kelarutan dan Contoh Soal Penyelesaian. Dalam suatu larutan jenuh dari suatu elektrolit yang sukar larut, terdapat kesetimbangan antara zat padat yang tidak larut dan ion-ion zat itu yang larut. MxAy (s) ⇄ x My+(aq) + y Ax.

Soal Kimia tentang Kelarutan dan Hasil kelarutan serta Pembahasannya
7.9 x 10 -15. Fe (OH) 3. 6.3 x 10 -38. Pb (OH) 2. 2.8 x 10 -16. Mg (OH) 2. 1.5 x 10 -11. Mn (OH) 2. 4.6 x 10 -14.

Cara Menentukan Rumus Ksp Suatu Senyawa Shorts Kimia Kelarutan Ksp Contohsoalkimia
Playlist Hasil Kali Kelarutan (KSP)https://www.youtube.com/watch?v=2VeNbLCqkeo&list=PLQ_4a1thWasOfRS4CERWeg_D4O-6tFekeJika teman-teman membutuhkan soal-soal.

Rumus hasil kali kelarutan (KSP) Ag2CrO4 dinyatakan sebag...
Dari rumus Ksp tersebut dapat ditentukan nilai kelarutannya dengan rumus berikut: Pengaruh Ion Senama terhadap Kelarutan. Penambahan ion senama dapat merubah molaritas zat. Sesuai asas Le Chatelier tentang pergeseran kesetimbangan apabila ke dalam larutan jenuh ditambahkan ion senama, maka kesetimbangan akan bergeser ke kiri (ke arah pereaksi.

Materi Kelarutan Dan Hasil Kali Kelarutan Homecare24
It is meaningless to compare the solubilities of two salts having different formulas on the basis of their Ks values. Example 17.2.2 17.2. 2. The solubility of CaF 2 (molar mass 78.1) at 18°C is reported to be 1.6 mg per 100 mL of water. Calculate the value of Ks under these conditions.