
CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or Nonpolar,Octet Rule
To draw the Lewis structure of CO2, we first need to determine the number of valence electrons in each atom. Carbon has 4 valence electrons, while each oxygen atom has 6 valence electrons. In total, CO2 has 16 valence electrons. Next, we need to arrange the atoms in the molecule. Carbon is the central atom in CO2, with each oxygen atom bonded.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or Nonpolar,Octet Rule
Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. The carbon-oxygen ratio in a CO 2 molecule is 1:2. Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom.
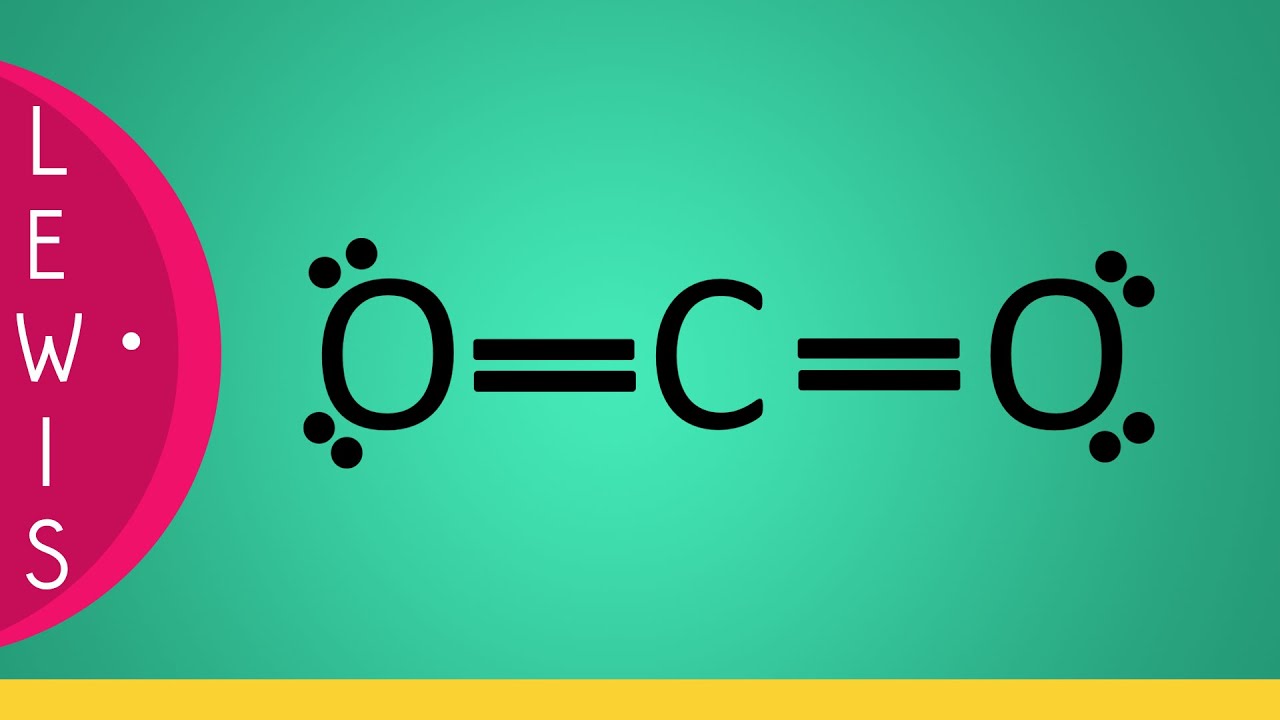
CO2 Lewis Structure YouTube
The CO 2 Lewis structure depicts the molecular arrangement of carbon dioxide, which is composed of one carbon atom and two oxygen atoms. Within the CO 2 Lewis structure, the carbon atom is surrounded by two double bonds, with each oxygen atom attached to it. Additionally, there are two lone pairs on each oxygen atom. To draw a CO 2 Lewis structure, begin by sketching out a rough structure of.

MENGGAMBAR STRUKTUR LEWIS CO2BANK SOAL KE 9 YouTube
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

CO2 Molecular Geometry Science Education and Tutorials
The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon.

What is the Lewis Dot structure for CO2 (Carbon dioxide)?
A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).For the CO2 structure use the periodic table to find the total number.

So far, we’ve used 16 of the CO2 Lewis structure’s total 16 outermost valence shell electrons
For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom. As all the valence electrons of all the atoms are used, there are no lone pairs of electrons or non-bonding pairs of electrons in the molecule. To further understand the molecular geometry of CO2, let us quickly go through its hybridization.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or Nonpolar,Octet Rule
These electrons push away the bonded pairs of electrons,giving it its "V shape". In the case of carbon dioxide,however,the carbon atom has no lone pairs so there is no repulsion between the bonded pairs and lone pairs. For the second part of your question, the electrons are present in a 3D region around the nucleus of an atom.

CO2 (Carbon Dioxide) Lewis Dot Structure Science Trends
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.
Carbon dioxide, CO2, molecule model and chemical formula. Carbonic acid gas. Colorless gas. Ball
A green carbon capture and conversion technology offering scalability and economic viability for mitigating CO 2 emissions is reported. The technology uses suspensions of gallium liquid metal to reduce CO 2 into carbonaceous solid products and O 2 at near room temperature. The nonpolar nature of the liquid gallium interface allows the solid products to instantaneously exfoliate, hence keeping.

Carbon Dioxide Lewis Structure How to Draw the Lewis Structure for Carbon Dioxide YouTube
1.2.1 Lewis Structure of Diatomic Molecules. To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots. The Lewis symbols of some elements are shown here: Figure 1.2a The Lewis structures of aluminum, tin, nitrogen, chlorine and bromine

CO2 Lewis Structure, Drawing Method of CO2 Lewis Structure, Molecular Geometry of CO2
Step #1: Calculate the total number of valence electrons. Here, the given molecule is CO2 (carbon dioxide). In order to draw the lewis structure of CO2, first of all you have to find the total number of valence electrons present in the CO2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

CO2, Struktur Lewis, Bentuk Molekul, dan Kepolarannya YouTube
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram Techiescientist
Steps of drawing CO2 lewis structure Step 1: Find the total valence electrons in CO2 molecule. In order to find the total valence electrons in CO2 (carbon dioxide) molecule, first of all you should know the valence electrons present in carbon atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
:max_bytes(150000):strip_icc()/CO2LewisStructure-591c94063df78cf5fadfde77.png)
Lewis Structure Definition and Example
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide YouTube
I quickly take you through how to draw the Lewis Structure of CO2 (Carbon DiOxide). I also go over hybridization, shape and bond angles.